Hemolysin BL
January 2010
A Pervasive Pest
Bacillus cereus is a bacterial jack-of-all-trades. Seemingly, it's happy almost anywhere. It is common in soil, where it grows and reproduces. When times get tough, it forms a weather-resistant spore and waits for better conditions. These spores find their way onto food of all sorts, and from there into our digestive system. The bacterium can also live in the intestine, along with the many other species of bacteria that inhabit us. Some strains of Bacillus cereus, however, build toxins like hemolysin BL that attack intestinal cells. These strains are thought to be a widespread cause of food poisoning, but this has been hard to quantify, since the symptoms are mild and pass quickly, and often are not reported to doctors.Pore-forming Toxins
Hemolysin BL, like many other bacterial toxins, forms a pore through the membrane of cells, allowing ions and small molecules to leak out. Creating a pore with a soluble protein is a tricky proposition. The protein must be soluble enough that it can be built and exported from the bacterial cell, but then it must create a pore within the hydrophobic environment of the membrane. The solution used by many toxins is a switchblade mechanism. The portion of the toxin that penetrates the membrane is folded inside the soluble form of the protein, waiting to be deployed when the target cell is found.B-component
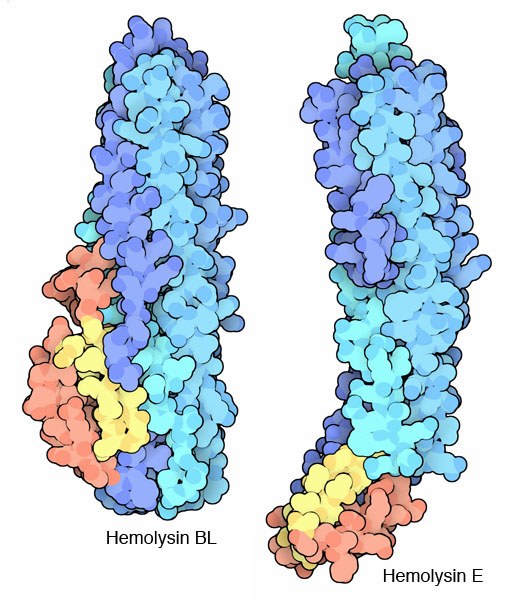
Researchers at NYSGXRC have solved the structure of hemolysin BL toxin in its soluble state, available in PDB entry 2nrj. In this form, the toxin is ready to seek out a target cell and build its deadly pore. Most of the protein chain forms a large bundle of alpha helices. A small hairpin loop, colored yellow here, is thought to be the penetration mechanism. When it finds its target cell, this loop will unfold and enter the membrane. Then, as several copies of the toxin bind side-by-side, a pore is formed. The active toxin is actually composed of three separate components: the B subunit shown here, and two L subunits that assist with the construction of the pore. The similar hemolysin E from Escherichia coli cells (entry 1qoy at the PDB) shows one possible step in this switchblade action, where the small domain that includes the penetration loop has opened up before binding.
Hemolysin BL (PDB entry 2nrj)
The B component of hemolysin BL is shown here with a ribbon diagram. The large alpha helical bundle is shown in magenta and the small beta hairpin, which is thought to be the portion that penetrates into the cell membrane, is colored yellow.
References
- Madegowda, M., Eswaramoorthy, S., Burley, S. K. and Swaminathan, S. (2008) X-ray crystal structure of the B component of Hemolysin BL from Bacillus cereus. Proteins 71, 534-540.
- Stenfors Arnesen, L. P., Fagerlund, A. and Granum, P. E. (2008) From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32, 579-606.



