Molecule of the Month: Hydrogenase
Hydrogenases use unusual metal ions to split hydrogen gas

Introduction
Splitting Hydrogen
Handling Hydrogen
Exotic Metal Complexes

Electron Carriers
Nickel-Iron Hydrogenase (PDB entry 2frv)
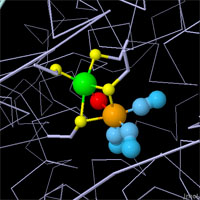
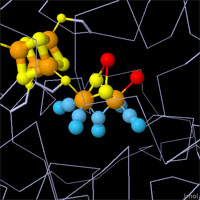
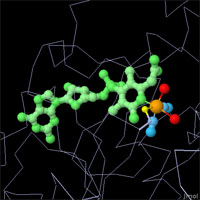
X-ray crystallography has revealed the active sites of all three types of hydrogenases,
but mysteries still remain. Hydrogen is too small to be seen in most crystallographic
experiments, so other methods must be used to discover the specifics of the reaction.
The active sites are all different, but they have compelling structural similarities.
All are centered around an iron atom with several unusual ligands, such as cyanide
ions and carbon monoxide. Each has another metal ion or cofactor to assist the iron
atom with the reduction/oxidation reaction. And they all use cysteine amino acids to
hold everything in place. Click on the images above to take an interactive look at
each active site.
You can get an up-to-date list of proteins related to Hydrogenase by clicking on any of
the accession codes in this Molecule of the Month, and then using the tab named
"Sequence Similarity" at the top of the page.
Topics for Further Discussion
- PDB entry 1hfe is an iron-iron hydrogenase from a different organism than the one shown here. It has a different set of ligands coordinated around the active site iron atoms. Can you see similarities and differences with the one shown here?
- Researchers are exploring the possibility of using hydrogenases for the production and use of hydrogen in technology. What challenges might they face?
Related PDB-101 Resources
- Browse Renewable Energy
- Browse Enzymes
References
- A.L. DeLacey, V.M. Fernandez, M. Rousset and R. Cammack (2007) Activation and inactivation of hydrogenase function and the catalytic cycle: spectroelectrochemical studies. Chemical Reviews 107, 4304-4330.
- K.A. Vincent, A. Parkin and F.A. Armstrong (2007) Investigating and exploiting the electrocatalytic properties of hydrogenases. Chemical Reviews 107, 4366-4413.
- M.R. Leach and D. B. Zamble (2007) Metallocenter assembly of the hydrogenase enzymes. Current Opinion in Chemical Biology 11, 159-165.
March 2009, David Goodsell
http://doi.org/10.2210/rcsb_pdb/mom_2009_3


