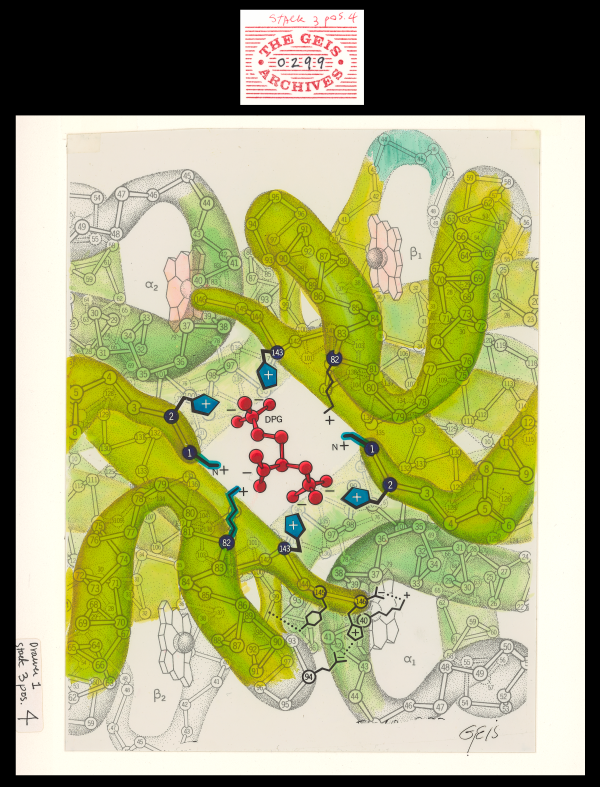
DPG-Hemoglobin Complex
Unknown Year, Unknown Dimensions

Geis illustrates the complex of DPG (2,3-diphosphoglycerate) with hemoglobin as a co-crystal. The charged amino acid residues stabilizing the complex with DPG are drawn in blue.
Used with permission from the Howard Hughes Medical Institute (www.hhmi.org). All rights reserved.

Related PDB Entry: 4L7Y
Experimental Structure Citation
To be published. Authors: Safo, M.K., Chowdhury, N.
About DPG-Hemoglobin Complex
2,3-diphosphoglycerate (DPG) is an inorganic phosphate produced in red cells. When DPG binds to deoxyhemoglobin, it acts to stabilize the low oxygen affinity state (T state) of the oxygen carrier. It fits neatly into the cavity of the deoxy- conformation, forming salt bridges with lysine and histidine residues in the ß subunits of hemoglobin. The R state, with oxygen bound to a heme group, has a different conformation and does not allow this interaction. By itself, hemoglobin has sigmoid-like kinetics, which makes easier another subunits’ binding.
Text Refrences
Reinhold Benesch, Ruth E. Benesch. (1967). The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 26(2):162–167.