Molecular Landscapes by David S. Goodsell
Coronavirus Life Cycle, 2020
Acknowledgement: David S. Goodsell, RCSB Protein Data Bank; doi: 10.2210/rcsb_pdb/goodsell-gallery-023
Integrative illustration for coronavirus outreach (2020) PLoS Biol 18: e3000815 doi: 10.1371/journal.pbio.3000815
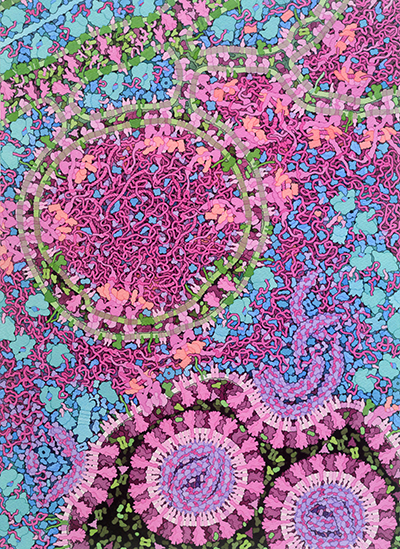
This illustration shows a cross section through a cell infected with a coronavirus such as SARS-CoV-2. It shows a time point when the virus is actively replicating, and new viruses are being created. The cell’s molecules are shown in blues and greens, and the viral molecules are shown in pinks and purples. The illustration integrates the current state of knowledge, but many aspects of the virus and its life cycle are still actively being studied, so portions of the painting are speculative. Additional resources for exploring coronavirus are available at PDB-101 and the main RCSB PDB site.

Several processes are depicted in the painting:
The painting shows one of the big mysteries of the coronavirus life cycle. The endoplasmic reticulum is extensively remodeled to form vesicles with two concentric membranes. The outer membrane is thought to be connected to the ER, but the inner membrane is thought to be sealed shut. The vesicle is thought to include viral RNA, some of which may be in double-stranded form. The function of these vesicles is still a subject of study, with many questions to be answered. Is the RNA replicating inside? If so, how do the building blocks get into the vesicle, and how does the final product get out?

Detail of ribosomes in the endoplasmic reticulum
A. Ribosome
B. Viral coding RNA
C. Translocation channel
D. Spike protein
E. Chaperonin BiP
F. Oligosaccharide transferase
G. Glucosidases

Detail of viral proteins
Numbers identify the viral non-structural proteins, so “3” is nsp3; sizes and shapes of each protein are based on current structural information, but the arrangement within the complex is largely speculative:
Nsp3 -- a multidomain protein that includes RNA-binding domains, a membrane-anchoring domain, and the papain-like protease
Nsp4 and 6 -- membrane-spanning proteins that remodel the ER membrane
Nsp5 -- main protease that cuts the viral polyproteins into functional pieces
Nsp7, 8 and 10 -- proteins involved in organizing the replicase complex
Nsp12 -- RNA-directed RNA polymerase creates new viral RNA strands
Nsp13 -- helicase separates strands in an RNA double helix
Nsp14 -- guanine N7-methytransferase includes an exoribonuclease involved in proofreading
Nsp15 -- uridylate-specific endoribonuclease breaks RNA, the function of which is still under study
Nsp16 -- 2’-O-methyltransferase is involved in formation of the RNA cap
Three structural proteins are also shown:
Nucleocapsid (N) condenses the viral genomic RNA
Membrane (M) protein works with N to package the RNA into the virion
Envelope (E) is involved in the process of budding
Several accessory proteins (p6, p7a, and p8a) are also shown. These are dispensable for replication of the virus, but are involved in the virulence of infection.
This version of the painting has a different coloring scheme, to highlight the double-membrane vesicles. Here, the membrane-bound components of the replicase are shown in green.
Selected References
Neuman BW, Buchmeier MJ (2016) Supramolecular architecture of the coronavirus particle. Advances in Virus Research 96, 1-27.
Neuman BW, Chamberlain P, Bowden F, Joseph J (2014) Atlas of coronavirus replicase structure. Virus Research 194, 49-66.
Knoops K, Kikkert M, van den Worm SHE, Zevenhoven-Dobbe JC, van der Meer Y, Koster AJ, Mommaas AM, Snijder EJ (2008) SARS-coronavirus replication is supported by a reticulvesicular network of modified endoplasmic reticulum. PLoS Biology 6: e226.
Goodsell DS (2011) Eukaryotic cell panorama. Biochemistry and Molecular Biology Education 39, 91-101.
More information on the painting is available in an article at PLoS Biology.