Molecular Models: Exploring the Structure of Insulin
In this activity you will make a paper model of Insulin, a peptide hormone that plays a critical role in our ability to use glucose from the food that we eat. You can compare the paper model to an atomic model of insulin displayed, in an online interactive, to learn further details about the structure and function of this molecule.
The activity is presented in 3 sections:
- About insulin - An introduction to the molecule
- Build a paper model of insulin - Template and instructions for making the paper model
- Explore the atomic structure of insulin - Interactive display of the atomic model of insulin and details about how its structure relates to its function
1. About Insulin?
Introduction:Insulin is a pancreatic hormone, released in response to high levels of blood glucose, for example after a meal. Insulin binds to specific receptors proteins on muscle, fat, liver and other cells in the body, promoting their glucose uptake for metabolism and/or storage. While many players participate in the glucose uptake process, the role of insulin is critical. In fact, when insulin is either missing or malfunctioning in an individual they are unable to use the glucose present in their blood and have a disease called Diabetes Mellitus.
Synthesis:Insulin is synthesized in the pancreas by specialized (beta) cells. Initially synthesized as preproinsulin, the first 24 residues function as a signal peptide, direct the protein to the rough endoplasmic reticulum (rER) and are cleaved. The remaining protein, called proinsulin, is folded in the ER lumen and three disulfide linkages are formed. The folded enzyme passes through the golgi-network where cellular peptidases (prohormone convertases, and carboxypeptidase E) act on proinsulin to cleave off 31 residues in the middle of the protein (referred to as C peptide). The mature insulin molecule retains two short peptides, N-terminal 30 residues (referred to as chain B) and C-terminal 21 residues (referred to as chain A). The 3 disulfide-linkages, described earlier, hold the two chains of insulin together. Mature insulin is stored in secretory vesicles within beta cells, ready to be released.
Secretion:Insulin is secreted at a basal level for normal function of different cells. However, in response to high blood glucose levels, a series of signal transduction steps are initiated, resulting in the release of a large amount (or bolus) of insulin from the stored secretory granules. During normal physiological function, insulin secretion is reduced or stopped in response to low glucose levels.
To learn more about the structure and functions of Insulin, see the Molecule of the Month features on Insulin and Insulin Receptor.
2. Build a Paper Model of Insulin
Use this PDF to build a paper model of Insulin.
About the insulin paper model:
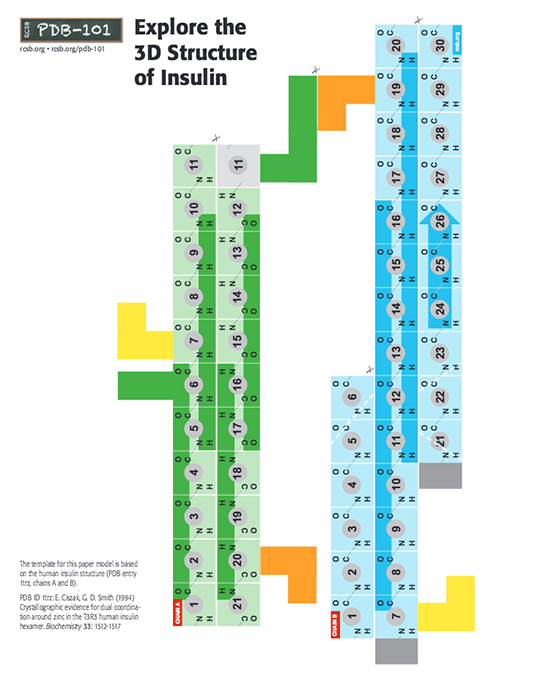
In the insulin model, chain A has 21 amino acids (colored green) and chain B has 30 amino acids (colored blue). Note the three disulfide-bridges holding the two chains together, colored yellow, green and orange.
Use this PDF to build a paper model of Insulin. As you build the model, you will see the primary, secondary, tertiary and quaternary structures of Insulin.
Folding Instructions
Follow the instructions in the video demonstrating how to build this insulin model.
Model limitations
- Each insulin molecule is made of 2 protein chains (called A and B in the model). The secondary structural regions shown in the model (especially of chain B) undergoes many changes - for example:
- The residues 1-8 are extended in a coil form in the T form of the structure (see chains A and B in PDB entry 1trz), while it forms part of the helix structure in the R form (see chains C and D in PDB entry 1trz). These conformational differences are not shown in the model but may be viewed in the JSmol online interactive shown below.
- The C-terminal residues in chain B of insulin change conformation upon binding to the insulin receptor. These conformational changes are not represented in this model.
- Electron micrographs of secretory granules in beta cell show that they are made of closely packed hexameric assemblies of insulin. Insulin hexamers consists of 6 molecules of insulin (Chain A and B), arranged as 3 dimers. Each dimer may be thought to assemble through side-by-side interaction of the C-terminal beta strand of the "A chain"(see paper model and video). The insulin hexamers may have all the "A chains" in T form making a T6 structure, or all of them in the R form, making an R6 structure. The hexameric structure seen in the PDB entry 1trz has 3 insulin molecules in the T and 3 in the R form, making a T3R3 structure.
3. Explore the Atomic Structure of Insulin
The atomic structure of insulin can be visualized using coordinates from the Protein Data Bank. The structure shown here is from PDB entry 1trz, which includes a hexameric form of human insulin. Three JSmol interactive views explore different aspects of the structure.
Insulin Structure
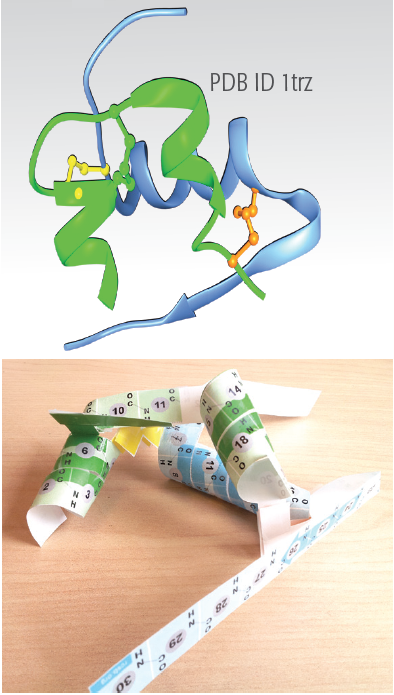
This JSmol shows the two chains of insulin, with the A chain in green and the B chain in blue. The three disulfide linkages are included in yellow, greenish yellow and pink, the same as used in the paper model. Hydrogen bonds are shown with dotted lines. You can use the buttons to show two different representations:
- a bond diagram that shows the atoms in the main polypeptide chains, along with hydrogen bonds. Sidechain atoms are not shown, except for the cysteine sidechains.
- a cartoon diagram that highlights the regions of the chains that form alpha helices.
You can also use the buttons to color the protein by atom type or by chain.
Note:
- Helices: If you look carefully, you'll see that the two small helices in the A chain (colored green) are not perfect alpha helices. Near the yellow disulfide bond, the first helix opens up a bit, and at the end of the second helix near the pink disulfide bond, the helix closes down a bit.
- Hydrogen bonds: Notice the different roles played by hydrogen bonds: stabilizing helices, organizing turns, and linking the two chains.
- Conformational flexibility: The long tail at the end of the B chain (ending in residue 30) is flexible and shows different conformations in different structural studies. These motions are thought to be important for interactions with the receptor.
Question:
- What is the functional role of the disulfides in this structure? Are they in a good place to perform this role?
Insulin Flexibility
This JSmol includes two of the insulin molecules from the hexamer in PDB entry 1trz. Use the buttons to switch between the two conformations (T and R states).
Note:
- More flexibility: Notice the large conformational change in the first eight amino acids of the B chain (shown in magenta).
Question:
- The T to R conformational changes are thought to be important for interaction with the insulin receptor. Can you come up with a reason for why this might be the case?
Insulin Hexamer
This JSmol shows the entire hexamer of insulin. For each insulin molecule, the A chain is in green and the B chain is in blue. Different shades of blue represent the two different conformations of the chain (T and R state). The hexamer is stabilized by two zinc ions, shown here in magenta. You can use the buttons to display the amino acid sidechains (pink and white) that interact with the zinc, as well as water molecules (red) and chloride ions (yellow green) that also interact with the zinc.
Note:
- Zinc interactions: Using the button, view the two zinc ions in the structure. Notice that they have very different interactions with the two different sides of the hexamer. Can you recognize the two different conformations of the B-chain shown in the previous JSmol?
- Dimers: The two neighboring B chains interact to form a small beta sheet and an insulin dimer (composed of T and R conformers). The easiest way to see this is in the cartoon representation.
- Hexamers: Use the "show atoms" button to see the entire hexamer structure. The hexameric form is used for storage, and the monomeric form is recognized by the receptor.
Question:
- If you wanted to make a long-lasting formulation of insulin, would you try to stabilize the hexamer or the monomer? How about a fast-acting insulin formulation, would that be more monomeric or hexameric?
References:
- Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes, Curr Diabetes Rev. 2013 Jan 1; 9(1): 25-53 (PMC3934755)
- Crystallographic evidence for dual coordination around zinc in the T3R3 human insulin hexamer. Journal: (1994) Biochemistry 33: 1512-1517