Molecule of the Month: Titin
The giant protein titin organizes the structure of muscle and gives them elasticity
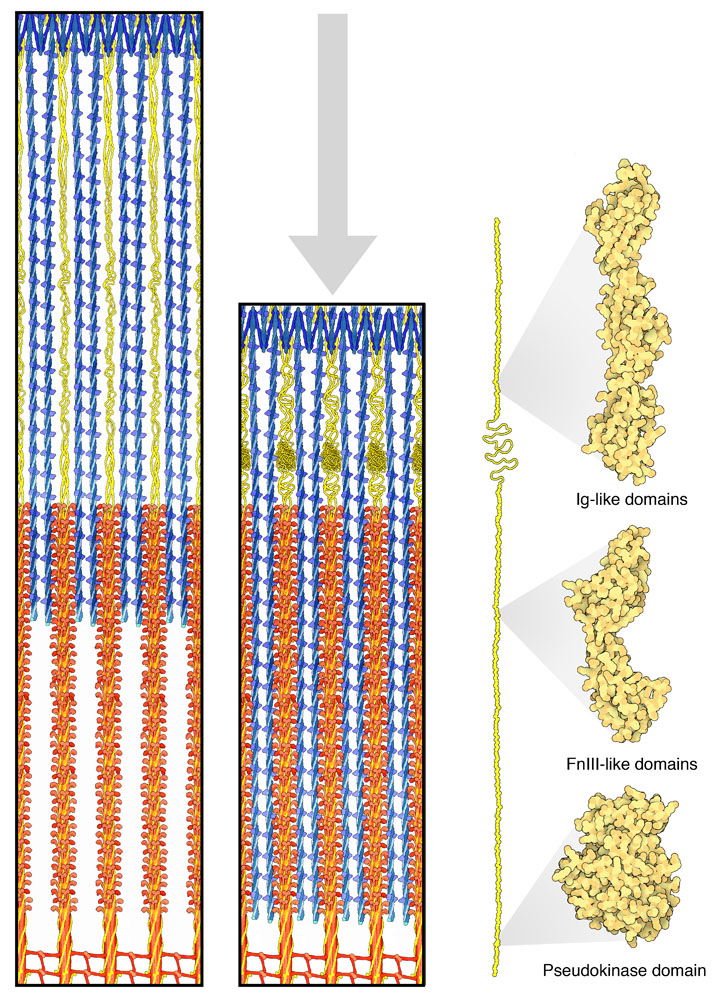
Introduction
Titanic Anatomy
Elastic Domains
Titin Partners
Exploring the Structure
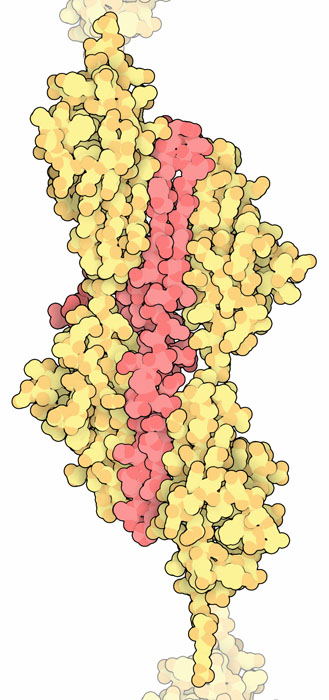
Titin I65-I70 (PDB entry 3b43)

The individual domains in titin are connected by short, flexible linkers, giving the whole structure the look of beads on a string. PDB entry 3b43 includes a short segment with six Ig domains. The curvy shape of the structure gives some idea of the flexibility of the titin chain. To explore this structure in more detail, click on the image for an interactive JSmol.
Topics for Further Discussion
- Many flexible proteins are constructed from tandem repeats of modular protein domains, although not as many as is found in titin. Try, for instance, searching for structures in the PDB for immunoglobulins, fibronectin, and cadherin.
Related PDB-101 Resources
- Browse Molecular Infrastructure
References
- W. A. Linke & N. Hamdani (2014) Gigantic business: Titin properties and function through thick and thin. Circulation Research 114, 1052-1068.
- L. C. Meyer & N. T. Wright (2013) Structure of giant muscle proteins. Frontiers in Physiology 4, 368.
- 3lpw: R. M. Bucher, D. I. Svergun, C. Muhle-Goll & O. Mayans (2010) The structure of the FnIII tandem A77-A78 points to a periodically conserved architecture in the myosin-binding region of titin. Journal of Molecular Biology 401, 843-853.
- 3b43, 2rik: E. von Castelmur, M. Marino, D. I. Svergun, L. Kreplak, Z. Ucurum-Fotiadis, P. V. Konarev, A. Urzhumtsev, D. Labeit, S. Labeit & O. Mayans (2008) A regular pattern of Ig super-motifs defines segmental flexibility as the elastic mechanism of the titin chain. Proceedings of the National Academy of Science USA 105, 1186-1191.
- 1ya5: P. Zou, N. Pinotsis, S. Lange, Y. H. Song, A. Popov, I. Mavridis, O. M. Mayans, M. Gautel & M. Wilmanns (2006) Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature 439, 229-233.
- 1tki: O. Mayans, P. F. van der Ven, M. Wilm, A. Mues, P. Young, D. O. Furst, M. Wilmanns & M. Gautel (1998) Structure basis of the activation of the titin kinase during myofibrillogenesis. Nature 395, 863-869.
May 2015, David Goodsell
http://doi.org/10.2210/rcsb_pdb/mom_2015_5


