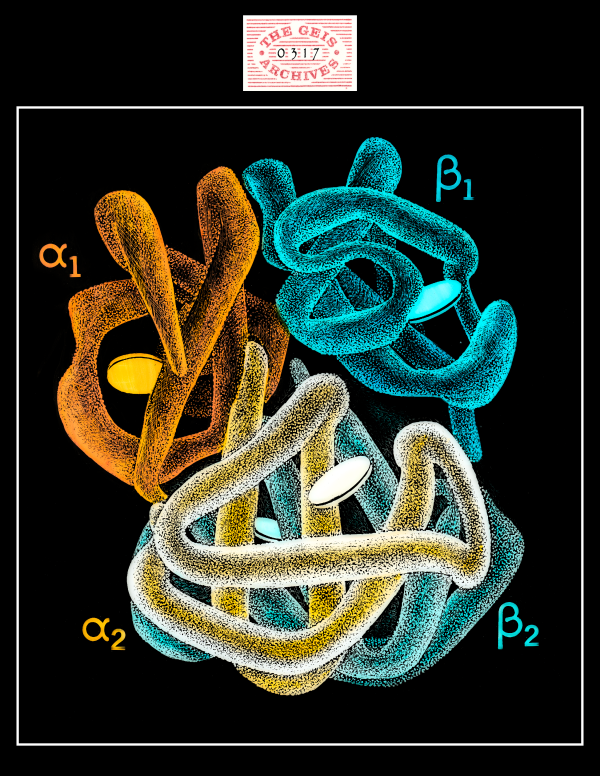
Hemoglobin S
1987

Geis illustrates the hemoglobin s molecule as four nearly symmetrically arranged subunits with a mutation present in the beta chain.
Used with permission from the Howard Hughes Medical Institute (www.hhmi.org). All rights reserved.

Related PDB Entry: 2HBS
Experimental Structure Citation
Ybe, J.A., Brodsky, F.M., Hofmann, K., Lin, K., Liu, S.H., Chen, L., Earnest, T.N., Fletterick, R.J., & Hwang, P.K. (1999) Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature, 399, 371-375. DOI: 10.1038/20708
About Hemoglobin S
Hemoglobin S is a rare form of the hemoglobin tetramer arising from a mutation in the gene encoding the beta subunit, which changes the normal glutamic acid at position 6 into a hydrophobic valine. As a result of this change in the protein structure, the deoxygenated form of hemoglobin S forms long fibers that distort red blood cells into a characteristic crescent or sickle shape. These distorted cells cause sludging and blockages in capillaries, leading to organ damage and painful crises typical of sickle cell disease--the first molecular disease to be described.
Text References
Dickerson, Richard Earl, and Irving Geis. Hemoglobin: Structure, Function, Evolution, and Pathology. Menlo Park, CA: Benjamin/Cummings Pub., 1983. Print.Richard, V., Dodson G. , and Mauguen, Y. (1993) Human Deoxyhemoglobin-2,3-Diphosphoglycerate Complex Low-Salt Structure at 2.5 A Resolution. J. Mol. Biol., 233, 270-274. Print.