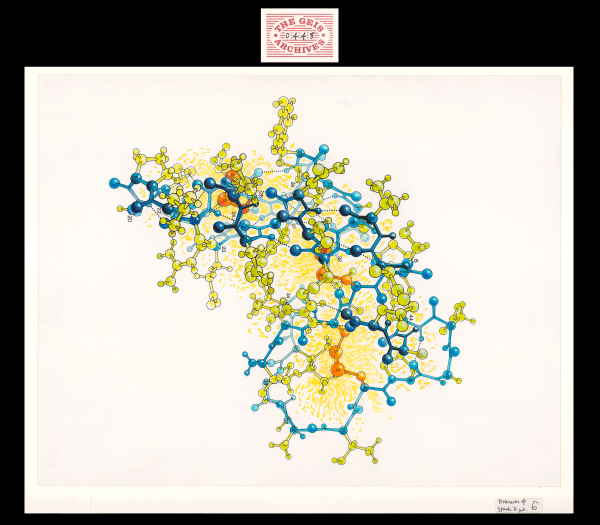
Crambin
1985, 14" x 11"

In Hendrickson and Teeter's molecular study in 1981, the crystal structure of crambin, a small seed storage protein, was determined based on the location of sulfur atoms in the protein. Using an artistic approach, Geis utilizes bright yellow shading and orange coloring to highlight the importance of these 6 sulfur atoms in this ball-and-stick representation. The backbone of the protein is depicted in blue.
Used with permission from the Howard Hughes Medical Institute (www.hhmi.org). All rights reserved.

Related PDB Entry: 1CRN
Experimental Structure Citation
Teeter, M. M. (1984). Water structure of a hydrophobic protein at atomic resolution: Pentagon rings of water molecules in crystals of crambin. Proc. Natl. Acad. Sci. USA, 81, 6014-6018.
About Crambin
Crambin is a small, hydrophobic plant seed protein from the Abyssinian cabbage that consists of 46 amino acids. Although its biological function is unknown, crambin has been extensively studied for its unique crystals and has been determined to very high resolution (0.48 Å as of 2011). The structure of crambin includes a bent backbone (colored in blue in the Jmol representation) that creates a groove, which is filled with side chains that stabilize the structure.
Initial Structure Determination References
Hendrickson, W.A. & Teeter, M.M. (1981). Structure of the hydrophobic protein crambin determined directly from the anomalous scattering of sulfur. Nature, 290, 107-113.
Schmidt, A., Teeter, M., Weckert, E., & Lamzin, V. S. (2011). Crystal structure of small protein crambin at 0.48 Å resolution. Acta. Crystallogr. Sect. F, 67, 424-428.