Alogliptin
Table 1. Basic profile of alogliptin
| Description | Oral anti-diabetic drug |
| Target(s) | Dipeptidyl peptidase-4 (DPP-4) |
| Generic | Alogliptin |
| Commercial Name | Nesina (United States, Canada), Vipidia® (United Kingdom) |
| Combination Drug(s) | Kazano (metformin & alogliptin; United States), Oseni (alogliptin & pioglitazone; United States) |
| Other Synonyms | Alogliptina, Alogliptine, Alogliptinum, SYR-322 |
| IUPAC Name | 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile |
| Ligand Code in PDB | T22 |
| 3D Structure of alogliptin bound to target protein DPP-4 | 3g0b |
|
|
|
Drug Information
Table 2. Chemical and physical properties (DrugBank). *Note: Predicted values may slightly vary from source to source.
| Chemical Formula | C18H21N5O2 |
| Molecular Weight | 339.39 g/mol |
| Calculated Predicted Partition Coefficient: cLogP* | 0.66 |
| Calculated Predicted Aqueous Solubility: cLogS* | -2.8 |
| Solubility (in water) | 0.58 mg/mL (sparingly soluble) |
| Predicted Topological Polar Surface Area (TPSA)* | 94 Å2 |
Drug Target
Alogliptin is an orally active, antidiabetic drug that works by inhibiting the enzyme dipeptidyl peptidase-4 (DPP-4) (Feng et al., 2007). In response to food intake,
endocrine cells in the gastrointestinal tract release incretin hormones, GLP-1 and GIP, to stimulate insulin secretion. Normally, DPP-4 degrades the incretin hormones within a few minutes of their release, thereby playing a key role in regulating the duration of incretin hormone function. Alogliptin, is a xanthine-based, non-substrate-like inhibitor of DPP-4. By blocking DPP-4 enzymatic activity, alogliptin increases the half-life of the incretin hormones, which in turn stimulates increased secretion of insulin by pancreatic β-cells and reduces secretion of glucagon by pancreatic α-cells. Collectively, these functions lower blood glucose levels. Since the incretins, GLP-1 and GIP, are only released after eating, DPP-4 inhibitors typically do not induce hypoglycemia (Aschner et al., 2006).
Learn more about DPP-4 here.
Drug-Target Complex
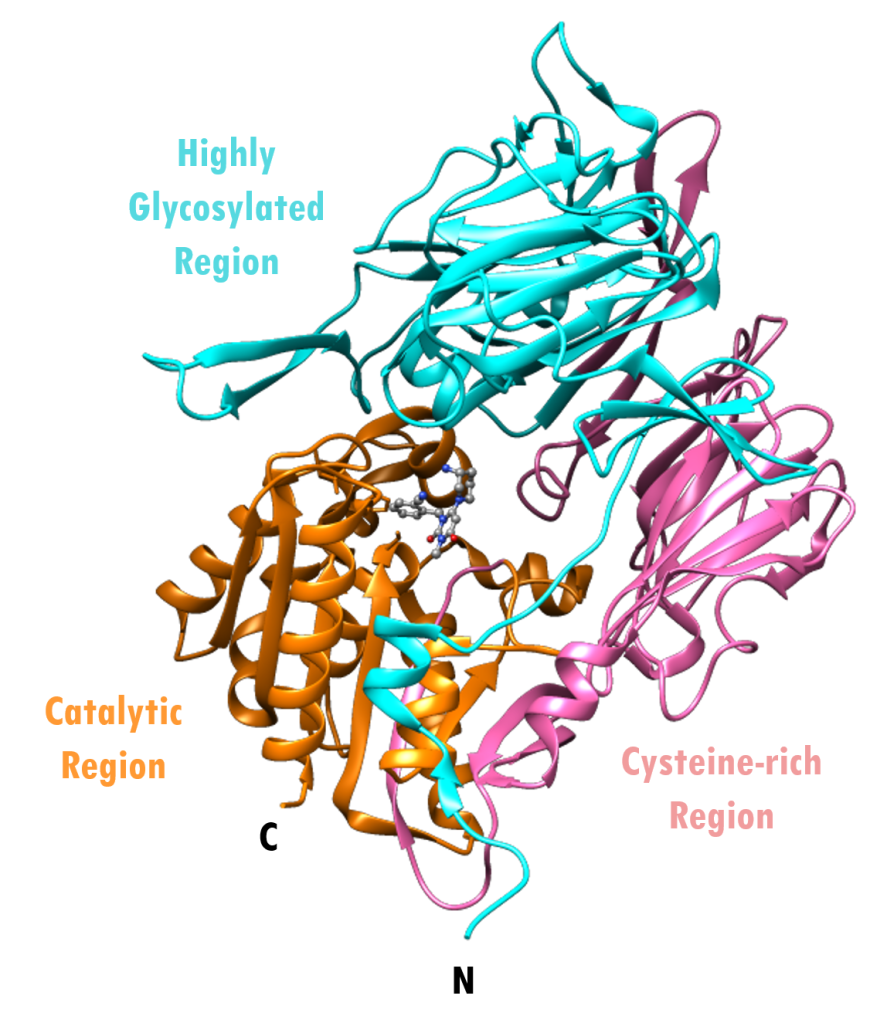
Each molecule of the DPP-4 enzyme is a transmembrane glycoprotein made up of 766 amino acids and consists of five regions:
- a cytoplasmic region (residues 1–6) not crystallized/not shown
- a trans-membrane region (residues 7–28) not crystallized/not shown
- a highly-glycosylated region (residues 29–323) colored cyan
- a cysteine-rich region (residues 324–551) colored pink
- a catalytic region (residues 552–766) colored orange
The overall structure of the extracellular portion of the enzyme, showing the highly glycosylated, cysteine-rich, and catalytic regions can be seen in Figure 2.
|
| Figure 2. Overall structure of human DPP-4 monomer complexed with alogliptin. The enzyme is shown in ribbon representation highlighting the N- and C-termini, and various regions of the protein - cysteine-rich region (pink), highly glycosylated region (cyan) and catalytic domain (orange). Alogliptin is shown in ball-and-stick representation (PDB ID: 3g0b; Zhang et al., 2011). |
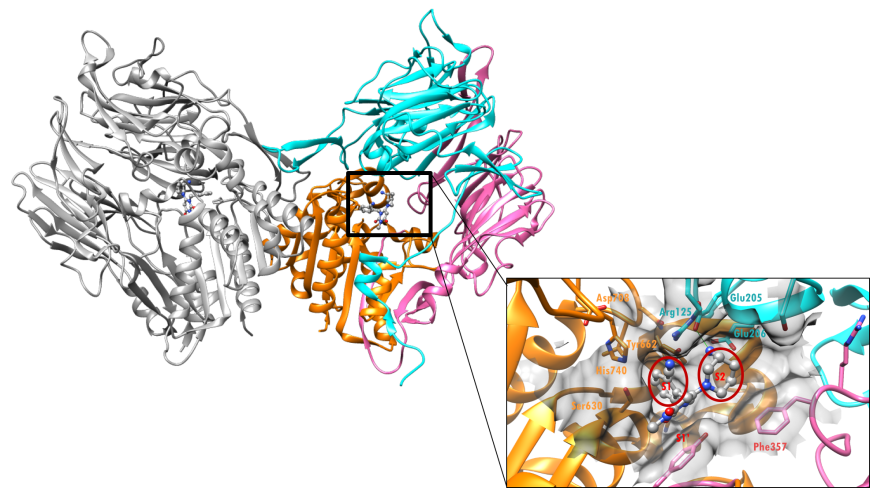
The DPP-4 enzyme functions as a dimer, composed of two copies of the same protein (Figure 3). Alogliptin is a xanthine-based, non-substrate-like inhibitor of DPP-4. This class of inhibitors binds non-covalently to the DPP-4 enzyme (Figure 3), and the S1 sub-pocket, of the active site, is occupied by an aromatic group (Figure 3 inset). When the enzyme binds to its natural substrates, or to substrate-like inhibitors, the S1 pocket is occupied by a proline, or a proline-like group, respectively.
|
| Figure 3. X-ray crystal structure of the DPP-4 dimer (ribbons) with bound alogliptin (ball-and-stick). The DPP-4 monomer on the right is color-coded by region as in Figure 2 and the monomer on the left is shown as a grey ribbon (PDB ID: 3g0b; Zhang et al., 2011). The surface of the active site of DPP-4 is shown in the inset. Alogliptin is shown in a ball-and-stick representation, color-coded by atom type (C: gray; N: blue; O: red). Selected residues in the active sites are shown as stick figures. |
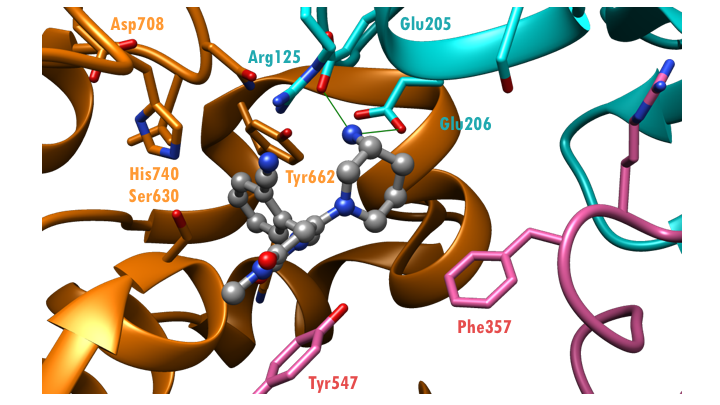
Closer examination of the X-ray structure of DPP-4 bound to alogliptin (PDB entry 3g0b, Zhang et al., 2011) reveals multiple interactions of the drug with its pharmacological target, DPP-4 (Figure 4). The cyanobenzyl group, on one end of alogliptin, occupies the hydrophobic S1 pocket (Figure 3, Figure 4), lined by several tyrosines, including Tyr662.The uracil ring, in the middle of the drug, engages in π-stacking interactions with Tyr547; while the aminopiperidine motif, on the other end of the drug, forms salt bridges with residues Glu205 and Glu206 (Figure 4). Together, these interactions account for the tight binding of alogliptin with DPP-4 (IC50 < 7 nm).
 |
 |
| Figure 4. Hydrogen bonding interactions (green lines) between alogliptin (ball-and-stick) and active site residues (stick figures) (PDB ID: 3g0b; Zhang et al., 2011). | Figure 5. Hydrogen bonding interactions (green lines) between Diprotin A (ball-and-stick) and active site residues (stick figures) (PDB ID: 1nu8; Thoma et al., 2003). |
Comparison of the co-crystal structures of DPP-4 with alogliptin (PDB ID 3g0b, Figure 4) and DPP-4 with its substrate, Diprotin A (Ile-Pro-Ile), (PDB ID 1nu8, Figure 5) reveals that alogliptin acts by occluding the DPP-4 active site and prevents binding of incretin hormones. Note that the side chain of Ser630, in the active site, is rotated away from the drug, in the DPP-4: alogliptin complex.
Pharmacologic Properties and Safety
Table 3. Pharmacokinetics: ADMET of alogliptin
| Features | Comment(s) | Source |
|---|---|---|
| Bioavailability (%) | 70% | (Capuano et al., 2013) |
| IC50 (nM) | 1 - <10 nM | BindingDB |
| Ki (nM) | N/A | N/A |
| Half-life (hrs) | 12.4-21.4 hours | (Capuano et al., 2013) |
| Duration of Action | 24 hours | (Cada et al., 2013) |
| Absorption | Human intestinal absorption | (DrugBank) |
| Transporter(s) | P-glycoprotein (P-gp) | (DrugBank) |
| Metabolism | Cytochrome p450 2D6 & 3A4 | (DrugBank) |
| Excretion | ~76% urine; ~13% feces | (Capuano et al., 2013) |
| AMES Test (Carcinogenic Effect) | probability 0.5595 (non AMES toxic) | (DrugBank) |
| hERG Safety Test (Cardiac Effect) | probability 0.5507 (weak inhibitor) | (DrugBank) |
| Liver Toxicity | Liver injury due to alogliptin is rare | (LiverTox) |
Drug Interactions and Side Effects
Alogliptin does not display any harmful effects on cardiac health - there is no significant increase in QT interval in individuals taking this drug (DrugBank). It is also not carcinogenic (DrugBank). Although there have been post-marketing reports of both fatal and non-fatal hepatic failure in patients, some of these reports contain insufficient evidence necessary to establish a probable cause.
Table 4. Drug interactions and side effects of alogliptin
| Features | Comment(s) | Source |
|---|---|---|
| Total Number of Drugs Interactions | 634 | (Drugs.com) |
| Major Drug Interactions | bexarotene and gatifloxacin | (Drugs.com) |
| Alcohol/Food Interaction(s) | moderate interaction with alcohol (ethanol) | (Drugs.com) |
| Disease Interaction(s) | pancreatitis (major), liver injury (moderate) and renal dysfunction (moderate) | (Drugs.com) |
| On-target Side Effects | Liver problems, pancreatitis, hypoglycemia | (Drugs.com) |
| Off-target Side Effects | Nasophargyngitis, headache, upper respiratory tract infection, allergic reactions, cold symptoms | (Drugs.com) |
| CYP Interactions | Unknown | (Capuano et al., 2013) |
Regulatory Approvals/Commercial
Nesina (alogliptin) was developed by Takeda, and approved by the US FDA in 2013. It is prescribed as an oral medication in 6.25, 12.5 or 25 mg per day to be taken with or without food (DrugBank). Patients with mild to extreme renal impairment are suggested to take a reduced dosage. The cost of alogliptin is $309.96 per month.
Alogliptin can be taken as a monotherapy or in conjunction with metformin (Kazano) or pioglitazone (Oseni). Kazano and Oseni were both manufactured by biopharmaceutical company Takeda Canada Inc.
In April 2016, the FDA released a statement highlighting concerns that Nesina (alogliptin), Kazano (alogliptin and metformin), and Oseni (alogliptin and pioglitazone) may increase the risk of heart failure, particularly in patients with heart or kidney disease.
Links
Table 5. Links to relevant resources
| DrugBank | http://www.drugbank.ca/drugs/DB06203 |
| Drugs.com | https://www.drugs.com/mtm/alogliptin.html |
| Food and Drugs Administration | https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022271s011lbl.pdf |
| National Institutes of Health (NIH) | https://livertox.nlm.nih.gov/Alogliptin.htm |
References
Alogliptin. Drugbank.ca. http://www.drugbank.ca/drugs/DB06203
Alogliptin. Livertox.nlm.nih.gov. https://livertox.nlm.nih.gov/Alogliptin.htm
Alogliptin. Pubchem.ncbi.nlm.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/Alogliptin
Alogliptin. - Affinity Data by PDB ID. Bindingdb.org. http://www.bindingdb.org/jsp/dbsearch/PrimarySearch_pdbids.jsp?pdbids_submit=Search&pdbids=3G0B
Aschner, P., Kipnes, M. S., Lunceford, J. K., Sanchez, M., Mickel, C., and Williams-Herman, D. E. (2006) Effect of the Dipeptidyl Peptidase-4 Inhibitor Sitagliptin as Monotherapy on Glycemic Control in Patients with Type 2 Diabetes. Diabetes Care 29, 2632-2637. https://doi.org/10.2337/dc06-0703
Cada, D. J., Levien, T. L., and Baker, D. E. (2013) Alogliptin. Hospital Pharmacy 48, 580-592. https://doi.org/10.1310/hpj4807-580
Capuano, A., Sportiello, L., Maiorino, M. I., Rossi, F., Giugliano, D., and Esposito, K. (2013) Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes Therapy – Focus on Alogliptin. Drug, Design, Development and Therapy 213, 989-1001. https://doi.org/10.2147/DDDT.S37647
Diabetes Medications Containing Saxagliptin and Alogliptin: Drug Safety Communication - Risk of Heart Failure. Fda.gov. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm494252.htm
Feng, J., Zhang, Z., Wallace, M. B., Stafford, J. A., Kaldor, S. W., Kassel, D. B., Navre, M., Shi, L., Skene, R. J., Asakawa, T., Takeuchi, K., Xu, R., Webb, D. R., and Takeuchi, K. (2007) Discovery of alogliptin: a potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV. Journal of Medicinal Chemistry 50, 2297-2300. https://doi.org/10.1021/jm070104l
Nesina (alogliptin) Side effects, Cost, Prescribing Information. RxEconsult. http://www.rxeconsult.com/healthcare-articles/-Nesina-alogliptin-Side-effects-Cost-Prescribing-Information-452/
Thoma, R., Loffler, B., Stihle, M., Huber, W., Ruf, A., and Hennig, M. (2003) Structural basis of proline-specific exopeptidase activity as observed in human dipeptidyl peptidase-IV. Structure 11, 947-959. https://doi.org/10.1016/S0969-2126(03)00160-6
Zhang, Z., Wallace, M. B., Feng, J., Stafford, J. A., Skene, R. J., Shi, L., Lee, B., Aertgeerts, K., Jennings, A., Xu, R., Kassel, D. B., Kaldor, S. W., Navre, M., Webb, D. R., and Gwaltney, S. L. (2011) Design and Synthesis of Pyrimidinone and Pyrimidinedione Inhibitors of Dipeptidyl Peptidase IV. Journal of Medicinal Chemistry 54, 510-524. https://doi.org/10.1021/jm101016w
September 2017, Avina S. Rami, Jennifer Jiang, Dr. Sutapa Ghosh ; Reviewed by Drs. Stephen K. Burley and Kathleen Aertgeerts
http://dx.doi.org/10.2210/rcsb_pdb/GH/DM/drugs/dppi/alogliptin




