DPP4
Dipeptidyl peptidase-4 (DPP-4) is a multifunctional serine protease, found anchored to the cell surface as a transmembrane glycoprotein, where it regulates the activity of bioactive peptides (Hiramatsu et al., 2003), and participates in cellular signaling. It is the target of several US FDA approved anti-diabetic medications, such as:
| Alogliptin | Linagliptin | Sitagliptin |
| Anagliptin | Saxagliptin | Vildagliptin |
These drugs block the degradation of incretins (GLP-1 and GIP) by DPP-4, and potentiate insulin secretion following food intake.
1. Function
Dipeptidyl peptidase-4 (also known as Adenosine deaminase (ADA) complexing protein 2 or T-cell activation antigen CD26) is a member of the prolyl oligopeptidase family of enzymes. DPP-4 has both enzymatic and non-enzymatic roles. The catalytic portion of DPP-4 carries out enzymatic cleavage of certain short bioactive peptides. The cysteine-rich and glycosylated regions of DPP-4 carry out the non-enzymatic functions, involved in cell signaling, immune regulation, inflammation, and tissue remodeling (Zhong et al., 2013). DPP-4 is also known to be cleaved from cell membranes in a cell specific manner that results in soluble circulating DPP-4. Recent studies have shown that circulating DPP-4 plays a role in obesity and cardiovascular diseases, and may represent an important molecular link between obesity, diabetes, and cardiovascular disease (Röhrborn et al., 2015).
DPP-4 plays a critical role in maintaining glucose homeostasis (Figure 1). It is responsible for inactivating incretins, such as glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). Secretion of these two intestinal hormones is triggered by food intake. As blood sugar levels rise after eating, the incretins potentiate glucose-dependent insulin secretion from pancreatic β-cells and inhibit glucagon secretion from pancreatic α-cells.
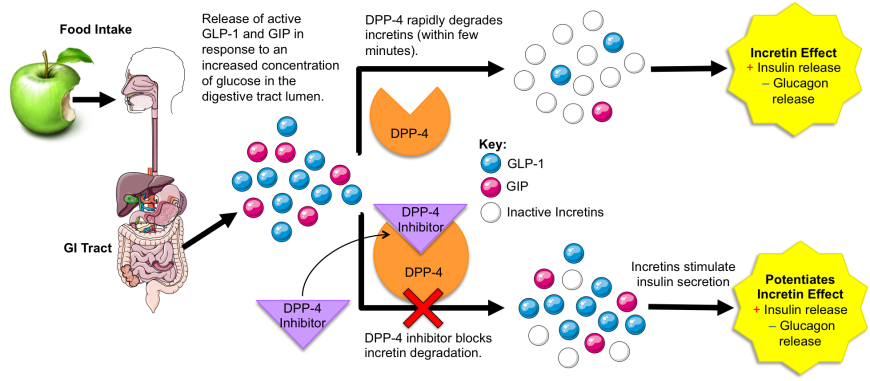
|
| Figure 1. DPP-4 function and mechanism of DPP-4 inhibitor action. |
DPP-4 rapidly degrades both GLP-1 and GIP (T1/2~4-5 minutes; Orskov et al., 1993), which helps prevent over production of insulin and hypoglycemia. Distributed throughout the skin, intestine, kidneys, liver, and other organs, DPP-4 not only cleaves incretins, but also other proteins with proline (P) or alanine (A) residues as the second amino acid (Wani et al., 2008). Some examples of the natural substrates of DPP-4 are enumerated below in Table 1, (Zhong et al., 2013):
Table 1: Examples of natural substrates of DPP4 and the effect of DPP4 action on them. PDB entries that include these substrates are also listed herein.
| Substrate of DPP-4 | Biological Function of Substrate | Effect of DPP-4 Action | Sequence of Cleaved peptide | PDB Entry |
|---|---|---|---|---|
| Glucagon like Peptide-1 (GLP-1) | Promotes insulin secretion | Inactivation | His-Ala- | 1d0r |
| Gastric inhibitory polypeptide (GIP) | Promotes insulin secretion | Inactivation | Tyr-Ala- | 2b4n, 1t5q |
| Gastrin-releasing Peptide (GRP) | Stimulates gastrin release | Inactivation | Val-Pro- | 7w3z |
| Neuropeptide NPY | Appetite control | Altered receptor specificity | Tyr-Pro- | 1r9n, 1ron |
| Neuropeptide YY | Appetite control | Altered receptor specificity | Tyr-Pro- | 2dez |
| Neuropeptide Substance P | Neurotransmitter and neuromodulator | Inactivation | Arg-Pro- | 2ks9 |
| Chemokine Rantes | Inflammatory response | Inactivation | Ser-Pro- | 1rtn, 1rto |
| Chemokine Eotaxin | Recruits eosinophils in allergic inflammation | Inactivation | Gly-Pro- | 2eot |
| C-X-C motif Chemokine 10 or IP-10 | Inflammatory response | Inactivation | Val-Pro- | 1lv9 |
2. Structure
DPP-4 is encoded by a member of the S9b gene family (also referred to as the “DPP-4 gene family”) (Han et al., 2015). The DPP-4 polypeptide chain is 766 residues in length and functions as a homodimer. X-ray crystallography revealed that each monomer consists of:
- An 8-bladed β-propeller domain (residues 61-495, colored green), which contains two substrate anchoring residues, Glu205, Glu206 (colored cyan)
- An α/β hydrolase domain (residues 39-55 and 497-766, colored magenta), which contains three catalytic residues, Ser630, His740, Asp708 (colored orange)
The domain organization of the DPP-4 dimer is shown in Figure-2 and a view of the enzyme active site is shown in the inset.
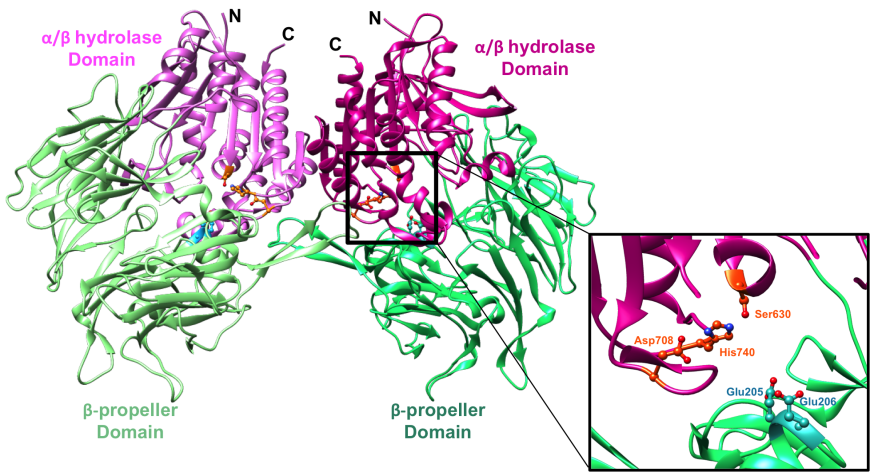
|
| Figure 2. Structure of the symmetric DPP-4 homodimer depicted in a ribbon representation. α/β hydrolase domain and the β-propeller domain of each monomer unit are colored in two shades of pink and green respectively (PDB ID 1r9m; Aertgeerts et al., 2004). The inset shows a closeup of the DPP-4 active site (corresponding to the right half) of the DPP-4 homodimer. The catalytic triad (Ser630, His740 and Asp708) is shown in orange and the anchoring Glu motif (Glu205/Glu206) in cyan. |
The α/β hydrolase domain consists of a central 8-stranded β-sheet sandwiched by 12 α-helices. (Thoma et al., 2003). The β-propeller domain has eight repeats of a structural motif, each made up of four antiparallel β-strands (Figure 3). The tertiary structure of the enzyme is stabilized by an extensive network of salt bridges, hydrogen bonds and van der Waals interactions (Thoma et al., 2003).
The β-propeller domain packs against the α/β hydrolase domain, and the catalytic triad (residues Ser630, His740 and Asp708) is located at the interface between the two domains (Aertgeerts et al., 2004). Both the α/β hydrolase and β-propeller domains participate in inhibitor binding. The catalytic serine S630 falls within the pentapeptide sequence Gly628-X-Ser630-Tyr631-Gly632, which is highly conserved across the α/β hydrolase protein family. This segment is essential for catalytic activity. The glutamate-rich loop (Glu205/Glu206) contributes to substrate recognition. It is also highly conserved within the DPP4-like protein family (Thoma et al., 2003). The anchoring Glu motif is positioned via salt bridge and hydrogen bonding interactions with Arg125 and other nearby residues.
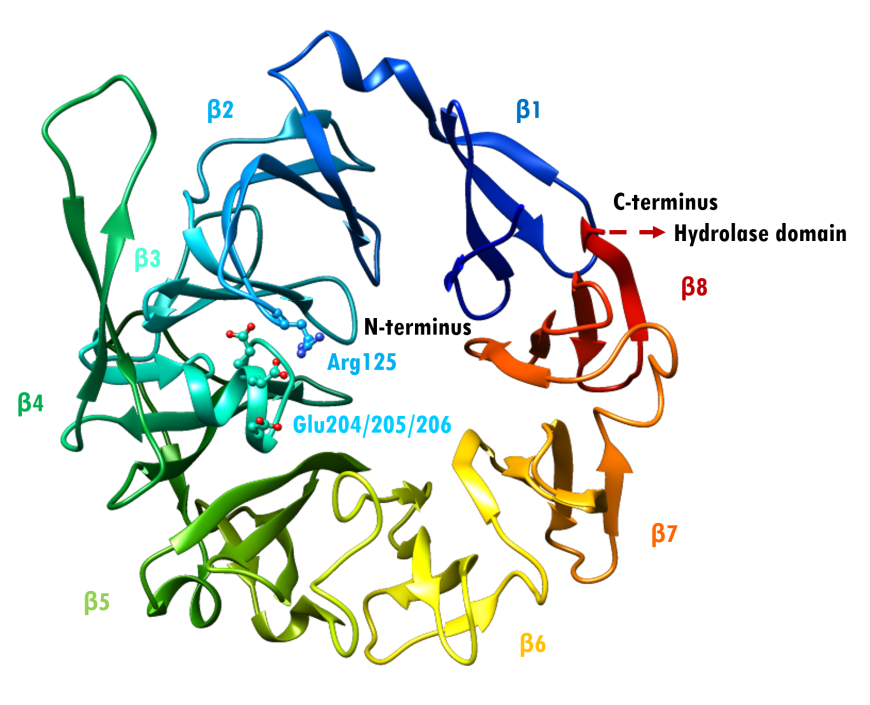
|
| Figure 3. Rainbow-colored ribbon representation of the β-propeller domain of DPP-4 (PDB ID 1r9m; Aertgeerts et al., 2004). Arg125 projects from blade β2. Glu205/206 project from blade β3. Source: Adapted from (Thoma et al., 2003). |
The enzyme active site is accessible via two openings: a cleft between the propeller and hydrolase domains (Figure 4, Upper) and funnel located in the center of the β-propeller domain (Figure 4, Lower) (Rasmussen et al., 2002). Both openings could provide access to the catalytic triad, as they have negative electrostatic potential and would attract the positively-charged N-terminus of the short bioactive peptide substrate (Rasmussen et al., 2002). Thoma et al. (2003) suggested that the cleft may enable large peptide substrates to access the active site, whereas the narrower tunnel formed by the β-propeller could serve as a conduit for exit of dipeptide cleavage products.

|
| Figure 4. Active site entry/exit conduits Upper: The cleft between the β-propeller (green) and the α/β hydrolase (pink). Domains. Lower: Narrower tunnel formed by the β-propeller domain (PDB ID 1r9m; Aertgeerts et al., 2004). |
Active Site
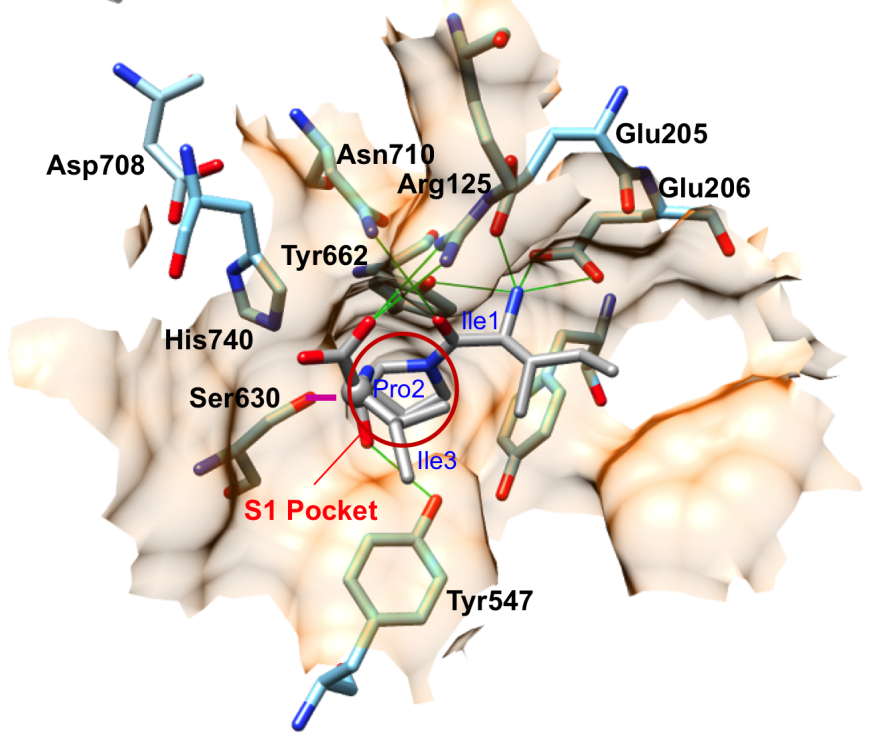
DPP-4 cleaves short bioactive peptide substrates immediately after the second residue from the N-terminus. Dipeptide cleavage is carried out by a catalytic triad (Ser630, Asp708, His740). Two nearby acidic residues (Glu205/Glu206) play important roles in positioning the substrate for catalysis. X-ray crystallography revealed how DPP-4 cleaves a tripeptide substrate (Ile-Pro-Ile), known as Diprotin-A (PDB ID 1nu8; Thoma et al., 2003). The N-terminus of the tripeptide is anchored in place via hydrogen bonding interactions with Glu205 and Glu206 (Figure 5). Pro2 of the Diprotin A makes multiple van der Waals interactions with the S1 hydrophobic pocket of DPP-4. Enzyme selectivity for cleavage immediately C-terminal to proline or alanine residues at position 2 substrate peptides is dictated by the shape and size of the S1 pocket, which is lined by Val656, Tyr631, Tyr662, Trp659, Tyr666, and Val711 (Figure 6). The bulkier proline occupies more of the S1 pocket than does alanine, which explains the higher affinity for peptides with proline versus alanine at the second position. Once the substrate is appropriately positioned, the activated serine nucleophile of the catalytic triad (Ser630) can attack the peptide bond between the second and third residue, and form a tetrahedral covalent intermediate. Thereafter, cleavage occurs and the N-terminal dipeptide and the remainder of the substrate oligopeptide are released. In PDB ID 1nu8, Diprotin A forms a tetrahedral intermediate, where the C atom of the carbonyl group of Pro2 in Diprotin A was found in a tetrahedral configuration (grey ball in Figure 5) covalently linked to Ser630. Although the tetrahedral intermediate is a high energy state with a short lifetime, this intermediate could have been kinetically trapped in the crystal structure. Figure 6 shows that the hydrophobic sidechains of Ile1 and Ile 3 of Diprotin A are in close proximity, and this hydrophobic interaction may stabilize the peptide in an conformation that is not suitable for reaction progression. Diprotein A is further held in this conformation via several hydrogen bonding interaction (green, Figure 5) with nearby active site residues. Glu205 and Glu206 interact with the N-terminus of the peptide. Glu205 makes salt bridge with Arg125, which in turn is hydrogen-bonded to the C-terminal carboxylate group of Diprotin A.
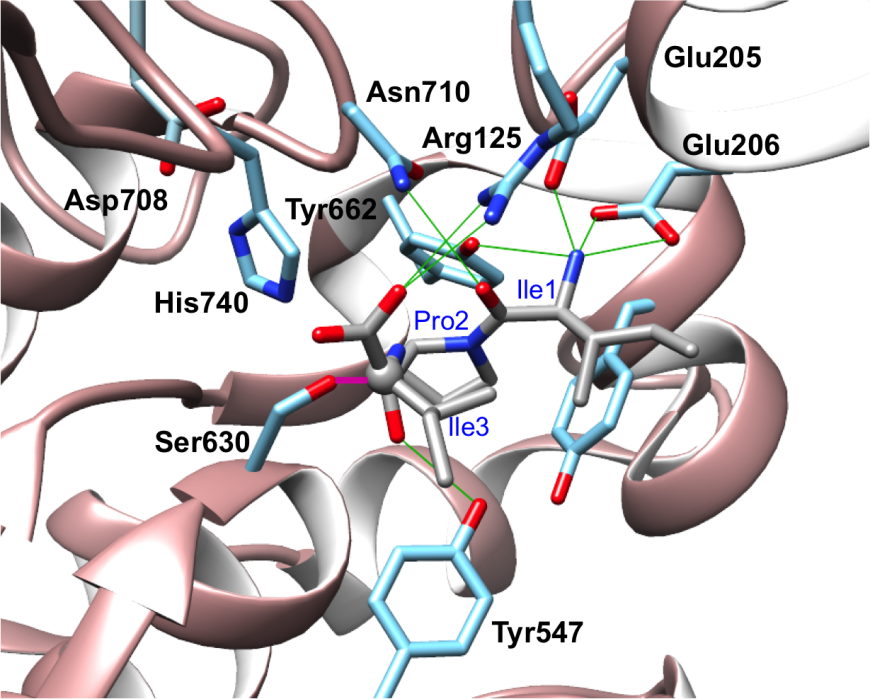
|
| Figure 5. Diprotin A (stick figure, in CPK colors) bound to the active site of DPP-4. Hydrogen bonding interactions between DPP-4 residues (blue stick figure) and Diprotin A are shown as green lines. The carbon or C atom (grey ball) of the carbonyl group of the scissile peptide (that is cleaved by the enzyme) in Diprotin A is covalently bound to Ser630 and is shown as a magenta line. (PDB ID 1nu8; Thoma et al., 2003). |
|
| Figure 6. Surface representation of the active site of DPP-4. Pro2 of Diprotin A (grey) occupies the S1 hydrophobic pocket. The magenta line represents the covalent bond between Ser630 of DPP-4 and Pro2 of Diprotin A. The catalytic serine S630 attacks the carbon atom of the substrate shown as a grey ball. (PDB ID 1nu8; Thoma et al., 2003) |
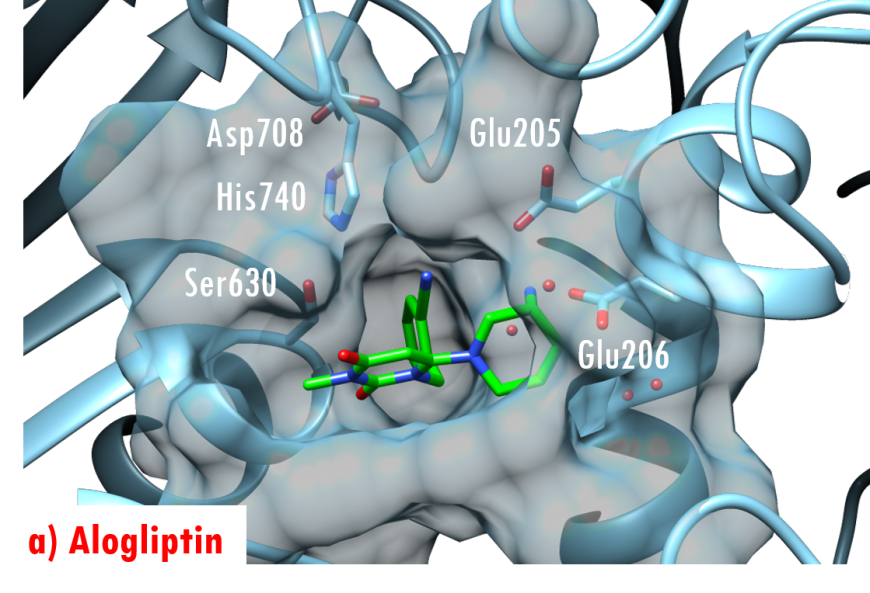
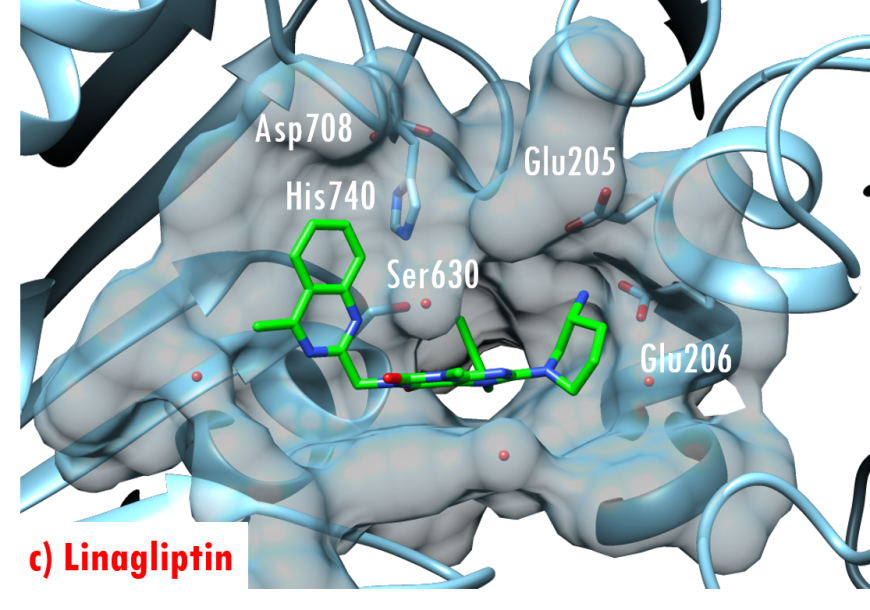
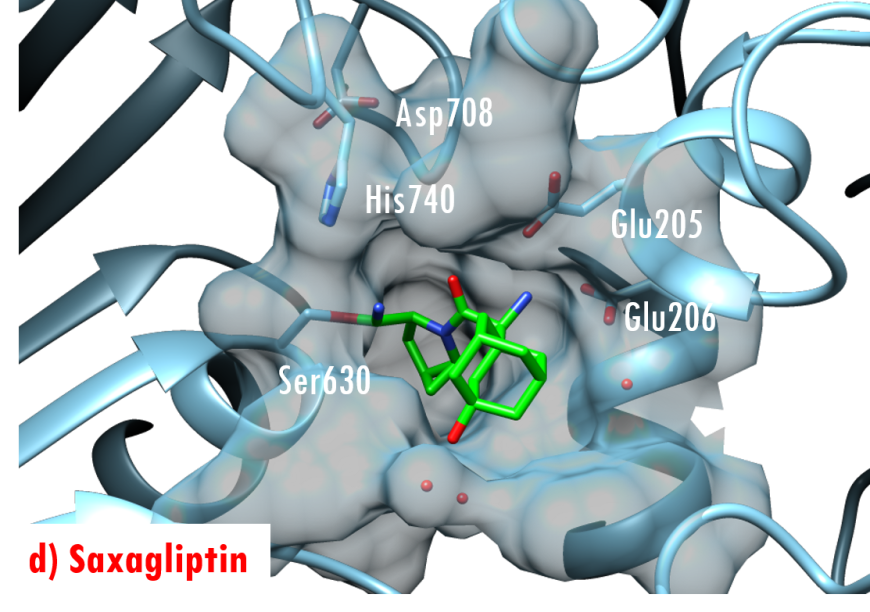
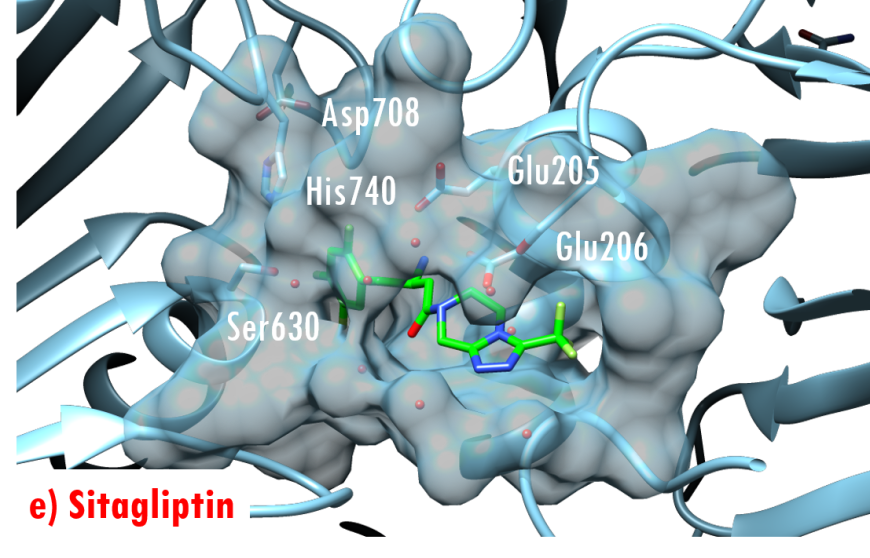
In the DPP-4/Diprotin A co-crystal structure (PDB ID 1nu8), the peptide bond between the second and third residues is not cleaved after formation of the tetrahedral intermediate, wherein the sidechain of the catalytic serine (Ser630) is covalently linked to the carbonyl carbon atom Pro2. Studies by Ogata et al. (1992) have shown that the sequence of the Gly628-X-Ser630-Tyr631-Gly632 pentapeptide is essential for DPP-4 catalysis. The DPP-4/Diprotin-A co-crystal structure shows that residues Gly628 and Gly632 are critical for correct positioning of the catalytic serine. All currently marketed anti-diabetic drugs targeting DPP-4 block substrate binding by making hydrogen bonds with the anchoring residues Glu205/Glu206 and occupying the S1 hydrophobic pocket (Figure 7).
 |
 |
 |
 |
 |
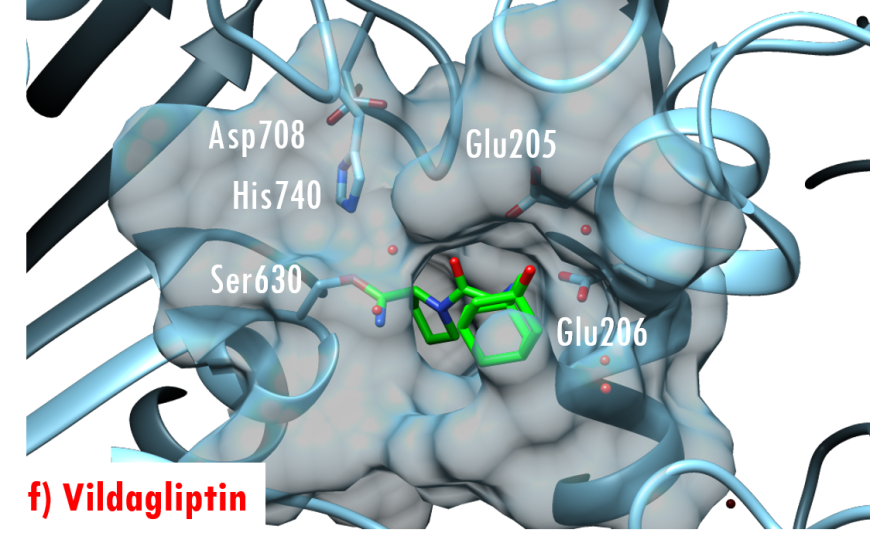 |
Figure 7. US FDA approved 'gliptin' drugs (green stick figures) mimic interactions between DPP-4 and its substrates. (a) Alogliptin, PDB ID 3g0b (Zhang et al., 2011); (b) Anagliptin, PDB ID 3wqh (Watanabe et al., 2015); (c) Linagliptin, PDB ID 2rgu (Eckhardt et al., 2007); (d) Saxagliptin, PDB ID 3bjm (Metzler et al., 2008); (e) Sitagliptin, PDB ID 1x70 (Kim et al., 2005); (f) Vildagliptin PDB ID 3w2t (Nabeno et al., 2013).
3. Pharmacologic Implications
DPP-4 inhibitors comprise a class of non-insulin anti-diabetic therapeutic interventions that enhance insulin secretion by pancreatic β-cells and act by blocking the enzymatic action of DPP-4. Oral administration of US FDA approved DPP-4 inhibitors, such as Saxagliptin, Vildagliptin, Anagliptin, etc., prolongs the half-lives and thus the action of GLP-1 and GIP, which in turn improves glycemic control with minimal risk of hypoglycemia (Havale and Pal, 2009).
4. Off-Target Considerations
The protein fibroblast activation protein-alpha (FAPα, Aertgeerts etal., 2005) is the closest relative of DPP-4 (~50% sequence similarity). Together with DPP-4, DPP-8, and DPP-9, FAP forms a family of post-proline dipeptidyl aminopeptidases, often called the DPP-4 family of proteins. The FAPα protein is highly expressed in epithelial cancers, playing a role in extracellular matrix remodeling, tumor growth, and metastasis. Both FAP and DPP-4 exhibit dipeptidase activity, but interestingly only FAP exhibits endopeptidase activity as well.
The dipeptidyl peptidase-8 (DPP-8) and dipeptidyl peptidase-9 (DPP-9) share high structural and functional similarity with DPP-4 (Liu et al., 2014). Both enzymes are ~25% identical to DPP-4 at the amino acid level. They do, however, differ from DPP-4 in terms of cellular locations. Both are cytosolic enzymes, whereas DPP-4 is processed by the Golgi apparatus and displayed on the surface of the cell where both GIP and GLP-1 act (Lankas et al., 2005).
Not surprisingly, a subset of DPP-4 inhibitors evaluated during pre-clinical development were found to suppress DPP-8 and/or DPP-9 activity, causing severe toxicities, including weakened T cell activation and proliferation, impaired preadipocyte differentiation (Han et al., 2015), enlarged lymph nodes, splenomegaly, etc. DPP-4 inhibitors in clinical use today exhibit little or no binding to either DPP-8 or DPP-9.
NB: Involvement of DPP-8 and DPP-9 in diabetes and obesity, if any, is not well understood at this time (Han et al., 2015).
References
Aertgeerts, K., Ye, S., Tennant, M. G., Kraus, M. L., Rogers, J., Sang, B. C., Skene, R. J., Webb, D. R., and Prasad, G. S. (2004) Crystal structure of human dipeptidyl peptidase IV in complex with a decapeptide reveals details on substrate specificity and tetrahedral intermediate formation. Protein Science 13, 412-421. https://doi.org/10.1110/ps.03460604
Aertgeerts, K., Levin, I., Shi, L., Snell, G., Jennings, A., Prasad, G., Zhang, Y., Kraus, M., Salakian, S., Sridhar, V., Wijnands, R., and Tennant, M. (2005) Structural and Kinetic Analysis of the Substrate Specificity of Human Fibroblast Activation Protein α. Journal of Biological Chemistry 280, 19441-19444. https://doi.org/10.1074/jbc.C500092200
Eckhardt, M., Langkopf, E., Mark, M., Tadayyon, M., Thomas, L., Nar, H., Pfrengle, W., Guth, B., Lotz, R., Sieger, P., Fuchs, H., and Himmelsbach, F. (2007) 8-(3-(R)-Aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a Highly Potent, Selective, Long-Acting, and Orally Bioavailable DPP-4 Inhibitor for the Treatment of Type 2 Diabetes. Journal of Medicinal Chemistry 50, 6450-6453. https://doi.org/10.1021/jm701280z
Han, R., Wang. X., Bachovchin, W., Zukowska, Z., and Osborn, J. W.(2015) Inhibition of Dipeptidyl Peptidase 8/9 Impairs Preadipocyte Differentiation. Scientific Reports 5, 1-11. https://doi.org/10.1038/srep12348
Havale, S. H., and Pal, M. (2009) Medicinal Chemistry Approaches to the Inhibition of Dipeptidyl Peptidase-4 for the Treatment of Type 2 Diabetes. Bioorganic & Medicinal Chemistry 17, 1783-1802. https://doi.org/10.1016/j.bmc.2009.01.061
Hiramatsu, H., Kyono, K., Higashiyama, Y., Fukushima, C., Shima, H., Sugiyama, S., Inaka, K., Yamamoto, A., and Shimizu, R. (2003) The structure and function of human dipeptidyl peptidase IV, possessing a unique eight-bladed -propeller fold. Biochemical and Biophysical Research Communications 302, 849–854. https://doi.org/10.1016/S0006-291X(03)00258-4
Kim, D., Wang, L., Beconi, M., Eiermann, G. J., Fisher, M. H., He, H., Hickey, G. J., Kowalchick, J. E., Leiting, B., Lyons, K., Marsilio, F., McCann, M. E., Patel, R. A., Petrov, A., Scapin, G., Patel, S. B., Roy, R. S., Wu, J. K., Wyvratt, M. J., Zhang, B. B., Zhu, L., Thornberry, N. A., and Weber, A. E. (2005) (2 R)-4-Oxo-4-[3-(trifluoromethyl)-5, 6-dihydro [1, 2, 4] triazolo [4, 3-a] pyrazin-7 (8 H)-yl]-1-(2, 4, 5-trifluorophenyl) butan-2-amine: a Potent, Orally Active Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. Journal of Medicinal Chemistry 48, 141-151 https://doi.org/10.1021/jm0493156%20
Lankas, G. R., Leiting, B., Roy, R. S., Eiermann, G. J., Beconi, M. G., Biftu, T., Chan, C. C., Edmondson, S., Feeney, W. P., He, H., Ippolito, D. E., Kim, D., Lyons, K. A., Ok, H. O., Patel, R. A., Petrov, A. N., Pryor, K. A., Qian, X., Reigle, L., Woods, A., Wu, J. K., Zaller, D., Zhang, X., Zhu L., Weber, A. E., and Thornberry, N. A. (2005) Dipeptidyl Peptidase IV Inhibition for the Treatment of Type 2 Diabetes: Potential Importance of Selectivity over Dipeptidyl Peptidases 8 and 9. Diabetes 54, 2988-2994. https://doi.org/10.2337/diabetes.54.10.2988%20
Liu, J., Huan, Y., Li, C., Liu, M., and Shen, Z. (2014) Establishment of a Selective Evaluation Method for DPP4 Inhibitors Based on Recombinant Human DPP8 And DPP9 Proteins. Acta Pharmaceutica Sinica B 4, 135-140. https://doi.org/10.1016/j.apsb.2013.12.007
Metzler, W. J., Yanchunas, J., Weigelt, C., Kish, K., Klei, H. E., Xie, D., Zhang, Y., Corbett, M., Tamura, J. K., He, B., Hamann, L. G., Kirby, M. S., and Marcinkeviciene, J. (2008) Involvement of DPP-IV catalytic residues in enzyme-saxagliptin complex formation. Protein Science 17, 240-250. https://doi.org/10.1110/ps.073253208%20
Nabeno, M., Akahoshi, F., Kishida, H., Miyaguchi, I., Tanaka, Y., Ishii, S., and Kadowaki, T. (2013) A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochemical and Biophysical Research Communications 434, 191-196. https://doi.org/10.1016/j.bbrc.2013.03.010
Ogata, S., Misumi, Y., Tsuji, E., Takami, N., Oda, K., and Ikehara, Y. (1992) Identification of the active site residues in dipeptidyl peptidase IV by affinity labeling and site-directed mutagenesis. Biochemistry 31, 2582–2587. https://doi.org/10.1021/bi00124a019
Orskov, C., Wettergren A., and Holst, J. J. (1993) Biological Effects and Metabolic Rates of Glucagon-like Peptide-1 7-36 Amide and Glucagon-like Peptide-1 7-37 in Healthy Subjects are Indistinguishable. Diabetes 42, 658-661. https://doi.org/10.2337/diab.42.5.658
Rasmussen, H. B., Branner, S., Wiberg, F. C., and Wagtmann, N. (2002) Crystal Structure of Human Dipeptidyl Peptidase IV/CD26 in Complex with a Substrate Analog. Nature Structural Biology 10, 19-25. https://doi.org/10.1038/nsb882
Röhrborn, D., Wronkowitz, N., and Eckel, J. (2014) DPP4 in Diabetes. Frontiers in Immunology 6, 386. https://doi.org/10.3389/fimmu.2015.00386
Thoma, R., Löffler, B., Stihle, M., Huber, W., Ruf, A., and Hennig, M. (2003) Structural Basis of Proline-Specific Exopeptidase Activity as Observed in Human Dipeptidyl Peptidase-IV. Structure 11, 947-959. https://doi.org/10.1016/S0969-2126(03)00160-6
Wani, J. H., John-Kalarickal, J., and Fonseca, V. A. (2008) Dipeptidyl Peptidase-4 as a New Target of Action for Type 2 Diabetes Mellitus: A Systematic Review. Cardiology Clinics 26, 639-648. https://doi.org/10.1016/j.ccl.2008.06.008
Watanabe, Y. S., Yasuda, Y., Kojima, Y., Okada, S., Motoyama, T., Takahashi, R., and Oka, M. (2015). Anagliptin, a potent dipeptidyl peptidase IV inhibitor: its single-crystal structure and enzyme interactions. Journal of Enzyme Inhibition and Medicinal Chemistry 30, 981-988. https://doi.org/10.3109/14756366.2014.1002402
Zhang, Z., Wallace, M. B., Feng, J., Stafford, J. A., Skene, R. J., Shi, L., Lee, B., Aertgeerts, K., Jennings, A., Xu, R., Kassel, D. B., Kaldor, S. W., Navre, M., Webb, D. R., and Gwaltney, S. L. (2011) Design and Synthesis of Pyrimidinone and Pyrimidinedione Inhibitors of Dipeptidyl Peptidase IV. Journal of Medicinal Chemistry 54, 510-524. https://doi.org/10.1021/jm101016w
Zhong, J., Rao, X., and Rajagopalan, S. (2013) An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis, 226, 305-314. https://doi.org/10.1016/j.atherosclerosis.2012.09.012
April 2017 , Jennifer Jiang, Dr. Sutapa Ghosh ; Reviewed by Drs. John Kozarich, Stephen K. Burley, and Kathleen Aertgeerts
http://dx.doi.org/10.2210/rcsb_pdb/GH/DM/drugs/dppi/dpp4



