Semaglutide
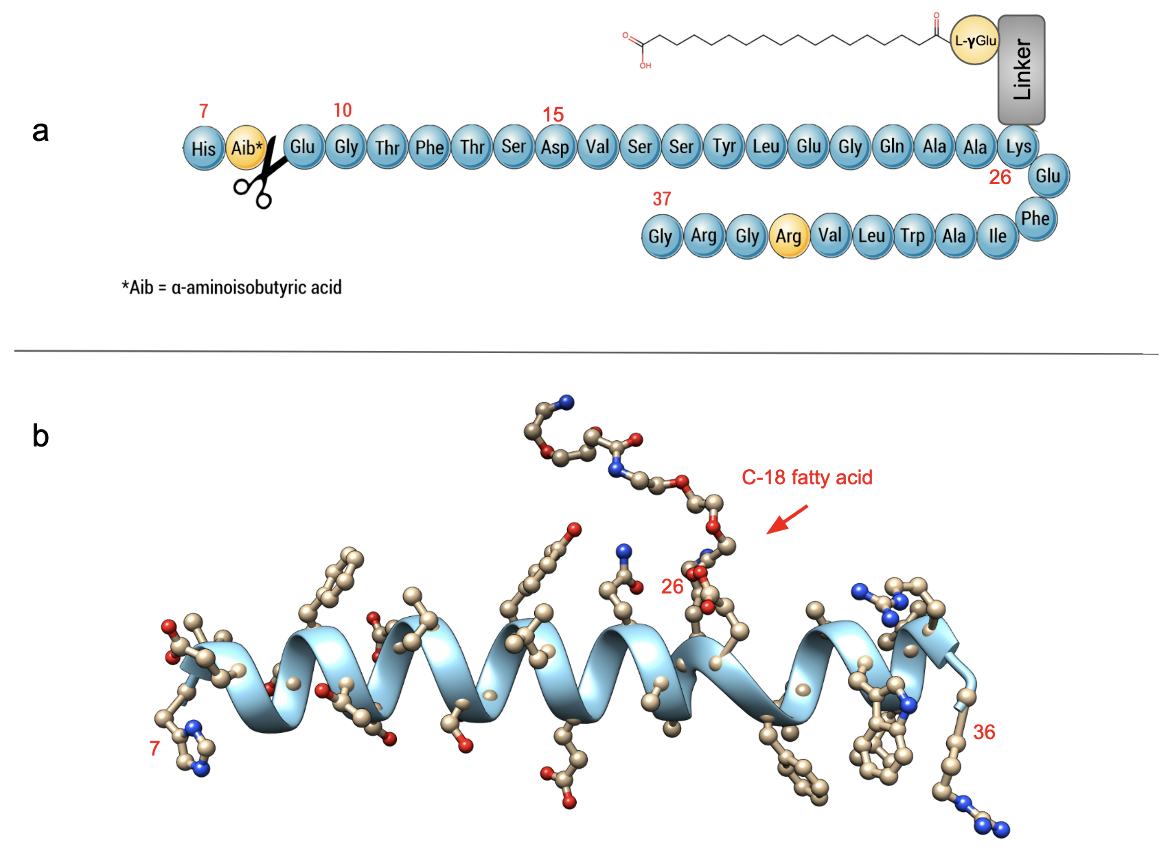
Semaglutide is a long acting GLP-1R agonist that is administered subcutaneously, as a treatment of type 2 diabetes. Developed by Novo Nordisk it was approved by the US FDA in 2017. Sharing a 94% structural homology with native human GLP-1, Semaglutide is distinguished by three modifications: 1) amino-acid substitutions at position 8 (alanine to α-aminoisobutyric acid or Aib) and position 34 (lysine to arginine), and acylation of the lysine in position 26 with a spacer consisting of two 8-amino-3,6-dioxaoctanoic acid (ADO) moieties, a glutamic acid moiety, and a C-18 fatty di-acid side chain (Lau et al., 2015).
Table 1. Basic Profile of Semaglutide
| Description | Oral and injectable anti-diabetic drug |
| Target(s) | Glucagon-like peptide 1 receptor (GLP-1R) |
| Generic Name | Semaglutide |
| Commercial Name | Ozempic, Rybelsus, Wegovy |
| Combination Drug(s) | N/A |
| Other Synonym | N/A |
| IUPAC Name | Semaglutide is a synthetic peptide (see the amino acid sequence below) |
| 3D Structure of Semaglutide | Chain P in PDB ID 7ki0 |
|
| Figure 1. 2D and 3D structures of Semaglutide. a. The amino acid sequence of Semaglutide (PubChem) is numbered from 7 to 37. The sequence schematic shown is based on information presented in Knudsen and Lau, 2019. Amino acid modifications are highlighted in yellow and the DPP4 cleave site is indicated with the scissors. b. 3D structure of Semaglutide (PDB ID 7ki0, Zhang et al., 2021). Note: The C-18 fatty acyl chain shown here is partially disordered. Only atoms included in the structures are shown here. The receptor and G-protein chains are hidden here for clarity. Click here to view the full structure interactively. |
Drug Information
Table 2. Chemical and physical properties of Semaglutide
| Chemical Formula | C187H291N45O59 |
| Molecular Weight | 4113.641 g/mol |
| Calculated Predicted Partition Coefficient: cLogP | No data available |
| Calculated Predicted Aqueous Solubility: cLogS | Not determined |
| Predicted Topological Polar Surface Area (TPSA) | 1650 Å2 |
Drug Target
Semaglutide was developed as an oral or an injectable drug product.
The addition of the C-18 fatty acid moiety to Lys26 is hypothesized to increase the binding dose-response ratio due to an increased albumin affinity. The replacement of Ala8 with Aib did not significantly change the binding affinity, but did result in a decline in potency as a substrate for DPP4. Collectively, these modifications prolong the half-life of semaglutide to approximately 1 week, or 165 hours (Lau et al., 2015). The mechanism of action is the same as GLP-1, including stimulation of insulin secretion, slowing gastric emptying, and reducing postprandial glucagon secretion in a glucose-dependent fashion.
The first generation of GLP-1 therapies exhibit reduced susceptibility to enzymatic degradation, while providing effective glucose control, improved β-cell function, body weight loss and decreased systolic blood pressure in patients with T2D (Meier, 2012) The newly developed incretin mimetics, including albiglutide, dulaglutide and semaglutide, are designed to have extended pharmacokinetic profiles and efficacy suitable once-weekly administration.
Drug Target Complex
Belonging to the class B G-protein-coupled receptor, GLP-1R is a seven-transmembrane protein receptor (Figure 2). It consisting of:
- An extracellular N-terminus that binds the C-terminal part of the incretin analog
- A seven transmembrane helical domain (TMD) with the a-helices separated by three intracellular loops and three extracellular loops
- An intracellular C-terminus that is responsible for signaling
The EM structure of Semaglutide bound to the complete GLP-1R and G-proteins (Figure 2, PDB ID 7ki0, Zhang et al., 2021) shows some hydrophobic and many specific polar interactions between the peptidic drug and the GLP-1 receptor molecule. At the N-terminus, the His7 of Semaglutide forms a direct hydrogen bond with the side chain of Gln234 and a water mediated hydrogen bond with Tyr241 of the receptor. The Glu9 forms an ionic bond with Arg190 and a hydrogen bond with Tyr152. The Thr13 forms a hydrogen bond with Tyr197, while Asp15 forms an ionic bond with R299. The Ser14 and Ser17 of Semaglutide form a network of hydrogen bonds involving the receptor residues Asn300, Arg299, and Thr298. The Tyr19 of Semaglutide forms a hydrogen bond with Glu138 of the receptor and Gln17 of the peptide, while Glu21 of Semaglutide forms a hydrogen bond with Ser31 and an ionic bond with Arg299 of the receptor. Hydrogen bonds between the backbone atoms of Val33 of Semaglutide (with the receptors Arg121) and the ionic bonds between Semaglutide’s Arg36 and the receptor’s Glu68, contribute to stably binding the C-terminal portion of the Semaglutide peptide.
While several of the interactions listed here are common to those seen between GLP-1 and its receptor, overall there appears to be many more specific polar interactions between Semaglutide and the GLP-1 receptor. These may play a key role in the high increased half life of the Semaglutide-GLP-1R complex (165+ hours, compared to the ~13 hours in the case of Liraglutide).

|
| Figure 2. EM structure of GLP-1R (red) with bound Semaglutide (gold) (PDB ID 7ki0, Zhang et a., 2021). Location of the membrane, and G-proteins associated with the receptor are also shown. The insets show a close up of specific polar interactions between the Semaglutide and the GLP-1R. |
Pharmacologic Properties
Table 3. Pharmacokinetics: ADMET of Semaglutide
| Features | Comment(s) | Source |
| Bioavailability (%) | 89% following subcutaneous injection | DrugBank |
| IC50 (nM) | 0.38 nm | Lau et al., 2015 |
| Ki (nM) | N/A | N/A |
| Half-life (hrs) | 168 hours | DrugBank |
| Duration of Action | 46.1 hours (in pigs) | Lau et al., 2015 |
| Absorption | N/A | N/A |
| Transporter(s) | Albumin | DrugBank |
| Metabolism | Metabolized through proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid sidechain; metabolism was not restricted to one organ | Jensen et al., 2017 |
| Excretion | Urine and feces were most important routes of excretion, but the former is the primary route | Jensen et al., 2017 |
| AMES Test (Carcinogenic Effect) | Not mutagenic or clastogenic in a standard battery of tests | US FDA |
| hERG Safety Test (Cardiac Effect) | Shown to reduce cardiovascular disease (CVD) risk in type 2 diabetes patients with established and/or high risk of CVD. The frequency and rate of serious adverse events, including serious cardiac disorders, were lower in the semaglutide group than in the placebo group | Dalsgaard et al., 2017 |
| Liver Toxicity | No treatment effects on hepatic enzymes or transaminases were observed. Further investigation is warranted. | Kapitza, et al., 2015 |
Drug Interactions and Side Effects
Table 4. Drug interactions and side effects of Semaglutide
| Features | Comment(s) | Source |
| Total Number of Drugs Interactions | over 240 interactions | Drugs.com |
| Major Drug Interactions | bexarotene and gatifloxacin; No significant effect on the pharmacokinetics of metformin, warfarin, atorvastatin and digoxin | Drugs.com; Hausner et al., 2017 |
| Alcohol/Food Interaction(s) | moderate interaction with alcohol (ethanol) | Drugs.com |
| Disease Interaction(s) | suicidal behavior and ideation (major), thyroid cancer (major), GI adverse events (moderate), hypoglycemia (moderate), pancreatitis (moderate), and retinopathy (moderate) | Drugs.com |
| On-site Binding Side Effects | Weight loss, diarrhea and other adverse gastrointestinal events | Marso et al., 2016; Drugs.com |
| Off-site Binding Side Effects | higher cardiovascular risk, and a greater incidence of diabetic retinopathy complications (3% vs 1.8%) | Drugs.com |
| CYP Interactions | cytochrome P450 (CYP) enzymes and transporters are not expected to be inhibited or induced by semaglutide | Hausner et al., 2017 |
In studying the effects of semaglutide treatment on the pharmacokinetics metformin, warfarin, atorvastatin and digoxin, it was found that this GLP-1 analog did not affect such properties to a clinically relevant degree (Hausner et al., 2017). Therefore, these drugs can be administered concomitantly with semaglutide without dose adjustment. However, since semaglutide impacts gastric emptying, the dosage of any other drugs taken concomitantly should be appropriately monitored by health care providers.
Regulatory Approvals/Commercial
Semaglutide is a GLP-1 analog, developed in 2012 by Novo Nordisk, as a longer acting alternative to liraglutide. The US FDA approved an injectable version (brand name Ozempic) as an anti-diabetes treatment in the United States in 2017 and in the rest of the world in 2018.
In 2019 and 2020, a formulation of Semaglutide (brand name Rybelsus) was approved as a daily oral medication for treating diabetes, when used along with diet and exercise. In 2022 this medication was approved as a first-line treatment for diabetes.
In 2021 - 2022, a stronger dose, weekly injectable formulation of Semaglutide (brand name Wegovy) was approved by the US FDA as a medication for weight loss in patients with at least one weight related medical issue.
In 2023, the high demand and possible off-label of this medication to treat obesity led to supply shortages of Wegovy.
Although Semaglutide is shown to be effective in lowering weight, HbA1c levels, and reducing cardiovascular risk, there are many side effects of this medication that should be closely monitored. The medication may have an impact on embryo and fetal development (US FDA package insert).
Links
Table 5. Links to Relevant Resources
| DrugBank | https://go.drugbank.com/drugs/DB06655 |
| Drugs.com | https://www.drugs.com/semaglutide.html |
| Food and Drugs Administration | https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf |
| Liver Tox: National Institutes of Health (NIH) | https://www.ncbi.nlm.nih.gov/books/NBK548574/ |
References
Dalsgaard, N.B., Brønden, A., Vilsbøll, T., Knop, F.K. (2017) Cardiovascular safety and benefits of GLP-1 receptor agonists. Expert Opin Drug Saf.16, 351-363. https://doi.org/10.1080/14740338.2017.1281246
Hausner, H., Derving Karsbøl, J., Holst, A.G., Jacobsen, J.B., Wagner, F.D., Golor, G., Anderson, T.W. (2017) Effect of Semaglutide on the Pharmacokinetics of Metformin, Warfarin, Atorvastatin and Digoxin in Healthy Subjects. Clin Pharmacokinet. 56, 1391-1401. https://doi.org/10.1007/s40262-017-0532-6
Jensen, L., Helleberg, H., Roffel, A., van Lier, J.J., Bjørnsdottir, I., Pedersen, P.J., Rowe, E., Derving Karsbøl, J., Pedersen, M.L. (2017) Absorption, metabolism and excretion of the GLP-1 analogue semaglutide in humans and nonclinical species. Eur J Pharm Sci. 104, 31-41. https://doi.org/10.1016/j.ejps.2017.03.020
Kapitza, C., Nosek, L., Jensen, L., Hartvig, H., Jensen, C.B., and Flint A. (2015). Semaglutide, a Once-Weekly Human GLP-1 Analog, does not reduce the Bioavailability of the Combined Oral Contraceptive, Ethinylestradiol/Levonorgestrel. Journal of Clinical Pharmacology, 55(5): 497-504. https://doi.org/10.1002/jcph.443.
Knudsen, L.B., Lau, J. (2019) The Discovery and Development of Liraglutide and Semaglutide. Front Endocrinol (Lausanne). 10, 155. https://doi.org/10.3389/fendo.2019.00155
Lau, J., Bloch, P., Schäffer, L., Pettersson, I., Spetzler, J., Kofoed, J., Madsen, K., Knudsen, L.B., McGuire, J., Steensgaard, D.B., Strauss, H.M., Gram, D.X., Knudsen, S.M., Nielsen, F.S., Thygesen, P., Reedtz-Runge, S., and Kruse, T., (2015), Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide, Journal of Medicinal Chemistry 58, 7370-7380. https://doi.org/10.1021/acs.jmedchem.5b00726.
Marso, S.P., Bain, S.C., Consoli, A., Eliaschewitz, F.G., Jódar, E., Leiter, L.A., Lingvay, I., Rosenstock, J., Seufert, J., Warren, M.L., Woo, V., Hansen, O., Holst, A.G., Pettersson, J., Vilsbøll, T. (2016) SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 375, 1834-1844. https://doi.org/10.1056/nejmoa1607141
Meier, J.J. (2012). GLP-1 receptor Agonists for Individualized Treatment of Type 2 Diabetes Mellitus. Nature Reviews Endocrinology, 8, 728-42. https://doi.org/10.1038/nrendo.2012.140
Zhang, X., Belousoff, M.J., Liang, Y.L., Danev, R., Sexton, P.M., Wootten, D. (2021) Structure and dynamics of semaglutide- and taspoglutide-bound GLP-1R-Gs complexes. Cell Rep. 36, 109374. https://doi.org/10.1016/j.celrep.2021.109374
August 2023 Jennifer Jiang and Dr. Shuchismita Dutta; Reviewed by Dr. Joseph D. Ho
http://dx.doi.org/10.2210/rcsb_pdb/GH/DM/drugs/in/Semaglutide



