Molecule of the Month: Capturing Beta-Lactamase in Action
Researchers visualize the mechanism of antibiotic resistance
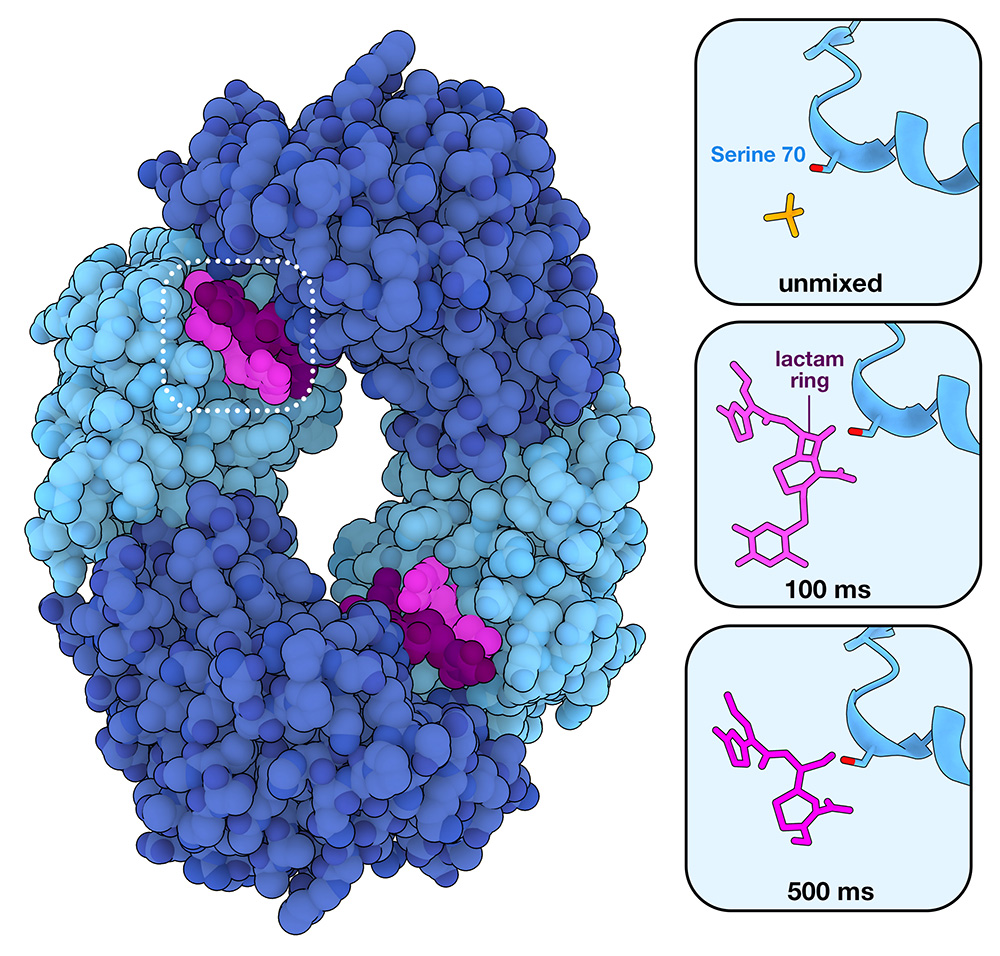
New technologies enable visualization of atomic-scale details of reactions as they occur
Antibiotic resistance in action
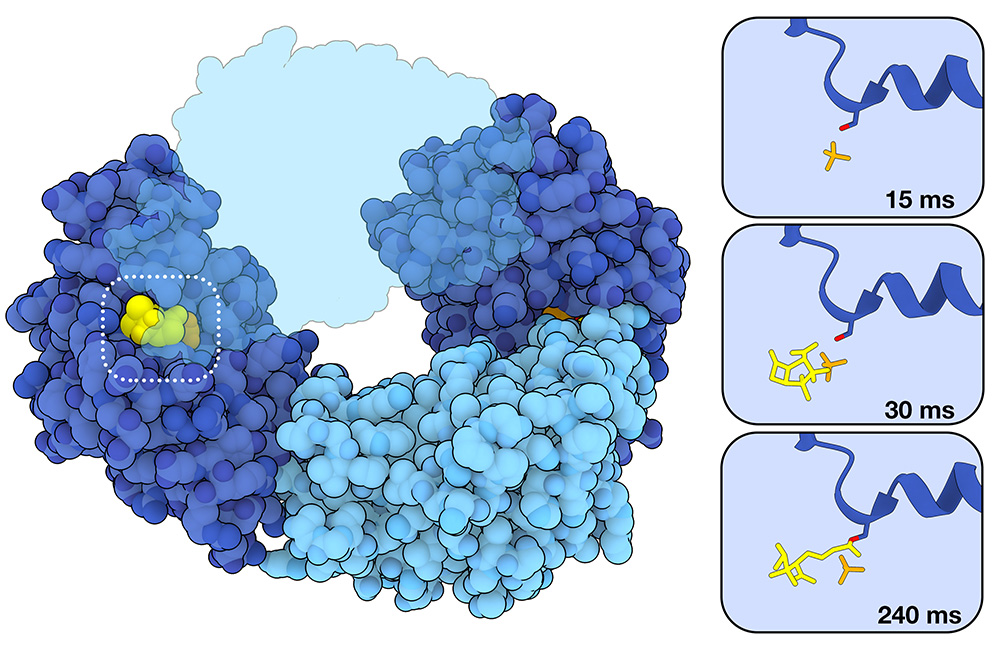
Capturing the action of inhibitors
Exploring the Structure
See β-lactamase in action
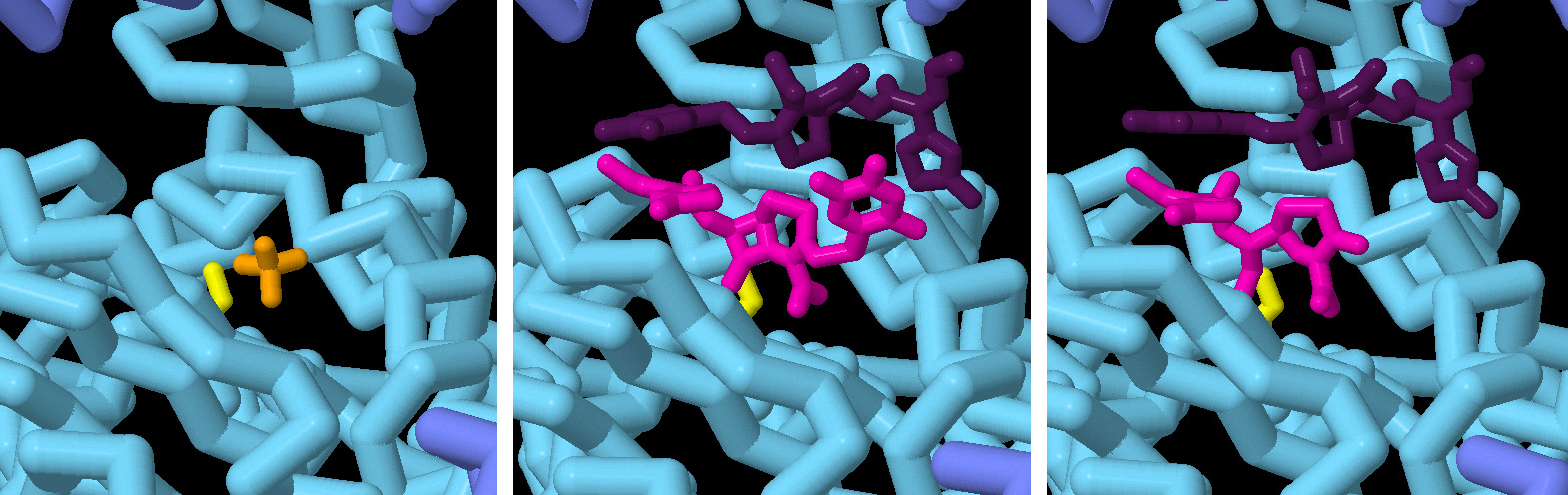
In this series of snapshots taken at different timepoints, the binding of an antibiotic (ceftriaxone) and subsequent cleavage of the β-lactam ring is revealed at atomic resolution. The catalytic serine of the enzyme is shown in yellow. In pink is the inactivated antibiotic, and in purple is a second antibiotic molecule that is in position to bind to the active site once it is emptied. Click over to the JSmol tab to view an animation of the structures.
Topics for Further Discussion
- Learn about a different β-lactamase, the New Delhi Metallo-beta-lactamase
- Read more about superbugs and antibiotic resistance.
- Different antibiotics have different ways of attacking microbes. Many bacterial antibiotics attack cell walls, like penicillin and vancomycin. Some anti-fungals, however, like actinomycin target DNA.
- Time-resolved crystallography techniques have also been used to capture light activatable proteins, such as photoactive yellow protein.
Related PDB-101 Resources
- Browse Antimicrobial Resistance
- Browse Drug Action
References
- 6B5X, 6B68, 6B69: Olmos JL Jr, Pandey S, Martin-Garcia JM, Calvey G, Katz A, Knoska J, Kupitz C, Hunter MS, Liang M, Oberthuer D, Yefanov O, Wiedorn M, Heyman M, Holl M, Pande K, Barty A, Miller MD, Stern S, Roy-Chowdhury S, Coe J, Nagaratnam N, Zook J, Verburgt J, Norwood T, Poudyal I, Xu D, Koglin J, Seaberg MH, Zhao Y, Bajt S, Grant T, Mariani V, Nelson G, Subramanian G, Bae E, Fromme R, Fung R, Schwander P, Frank M, White TA, Weierstall U, Zatsepin N, Spence J, Fromme P, Chapman HN, Pollack L, Tremblay L, Ourmazd A, Phillips GN Jr, Schmidt M. (2018) Enzyme intermediates captured “on the fly” by mix-and-inject serial crystallography. BMC Biol 16, 59.
- 8EBI, 8EBR, 8EC4: Malla TN, Zielinski K, Aldama L, Bajt S, Feliz D, Hayes B, Hunter M, Kupitz C, Lisova S, Knoska J, Martin-Garcia JM, Mariani V, Pandey S, Poudyal I, Sierra RG, Tolstikova A, Yefanov O, Yoon CH, Ourmazd A, Fromme P, Schwander P, Barty A, Chapman HN, Stojkovic EA, Batyuk A, Boutet S, Phillips GN Jr, Pollack L, Schmidt M. (2023) Heterogeneity in M. tuberculosis β-lactamase inhibition by Sulbactam. Nat Commun 14, 5507.
- Calvey GD, Katz AM, Schaffer CB, Pollack L. Mixing injector enables time-resolved crystallography with high hit rate at X-ray free electron lasers. Struct Dyn. 2016 Aug 29;3(5):054301.
- Wiedorn, M.O., Oberthür, D., Bean, R. et al. (2018) Megahertz serial crystallography. Nat Commun 9, 4025.
July 2025, Janet Iwasa
http://doi.org/10.2210/rcsb_pdb/mom_2025_7


