ATCase R-state
1988, 9 1/4" x 10 1/2"

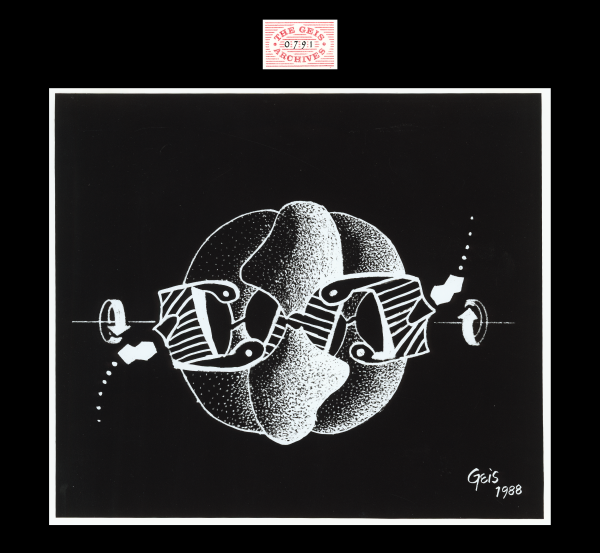
In this painting of ATCase R-state, ATP is pictured as a shaded double ring (adenine) binding to the regulatory dimers of the enzyme. Geis illustrates the movement of ATP towards the binding site with a trail of increasing spots. The binding of ATP to ATCase stabilizes the R-state and allows for high affinity substrate-binding.

Used with permission from the Howard Hughes Medical Institute (www.hhmi.org). All rights reserved.

Related PDB Entry: 2AT1
Experimental Structure Citation
Gouaux, J. E. & Lipscomb, W. N. (1990). Crystal structures of phosphonoacetamide ligated T and phosphonoacetamide and malonateligated R states of aspartate carbamoyltransferase at 2.8-Å resolution and neutral pH. Biochemistry, 29, 389-402.
About ATCase R-state
The R-state of ATCase is stabilized by the binding of ATP to the regulatory subunits. The binding of ATP leads to an increase in catalytic function of ATCase. In the Jmol representations of ATCase, the catalytic chains of ATCase are shown in blue while the regulatory chains are shown in yellow.
Text References
Lipscomb, W. N. & Kantrowitz, E. R. (2012). Structure and Mechanisms of Escherichia coli Aspartate Transcarbamoylase. Acc. Chem. Res. 45, 444-453.
Moffatt, B. A. & Ashihara, H. (2002). Purine and Pyrimidine Nucleotide Synthesis and Metabolism. The Arabidopsis Book 1:e0018. DOI: 10.1199/tab.0018
Wang, J., Eldo, J., & Kantrowitz, E. R. (2009). Structural Model of the R State of Escherichia coli Aspartate Transcarbamoylase with Substrates Bound. J. Mol. Biol. 371, 1261-1273.




