Porins
Introduction
Many antibiotic-resistant diseases are caused by gram-negative bacteria - e.g., Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Salmonella enterica. These bacteria have a cell wall that is comprised of inner and outer membranes, with periplasmic space in between. The outer membrane is impermeable to hydrophilic compounds and serves as the first line of defense against toxic compounds, such as antimicrobial molecules (Galdiero et al., 2012). It contains protein channels, called porins, that facilitate the inflow of nutrients and other compounds, including several classes of antibiotics (Pagès et al., 2008).
Learn more about types of bacteria and bacterial cell walls.
Normal Function of Porins
Porins were first identified in 1976 and named porins (Nakae, 1976). These open water-filled channels (Galdiero et al., 2012) allow a broad range of molecules to diffuse through the cell (Baslé et al., 2006). Besides water and nutrients, hydrophilic antibiotic molecules, such as β-lactams, tetracyclines, and fluoroquinolones also permeate through the outer membrane of gram-negative bacteria and reach their intracellular target sites using porins. For example, in the case of gram-negative bacteria, β-lactam antibiotics have to first cross the outer membrane to reach their target in the periplasmic space and promote bactericidal activity by targeting peptidoglycan cross-linking enzymes (transpeptidases and carboxypeptidases). Absence, blockage, or changes in the function of porin that reduce the ability of antibiotics to be transported into the periplasm can lead to resistance. The role of porins in the permeability and resistance to β-lactam antibiotics has been studied for many decades (Nikaido 1985) and will be discussed here.
Types of Porins
General bacterial porins allow the energy-independent transport of small molecules across the outer membranes of bacteria (Pagès et al., 2008). The most well-characterized members of this family (the classical porins) are the E. coli OmpF and OmpC porins.
Other examples in this class (CARD 2017, ARO:3004281 and ARO:3004282) include K. pneumoniae OmpK35, OmpK36, and OmpK37; Burkholderia pseudomallei Omp38; Moraxella catarrhalis M35; Neisseria gonorrhoeae porin PIB (por); Serratia marcescens Omp1; Vibrio cholerae OmpU, OmpT; and K. pneumoniae OmpA.
Here we examine the structures of E. coli OmpC, OmpF, and V. cholera OmpU to understand how they reduce uptake of β-lactams and other antibiotics.
Learn about other antibiotic resistance mechanisms.
Structure of Porins
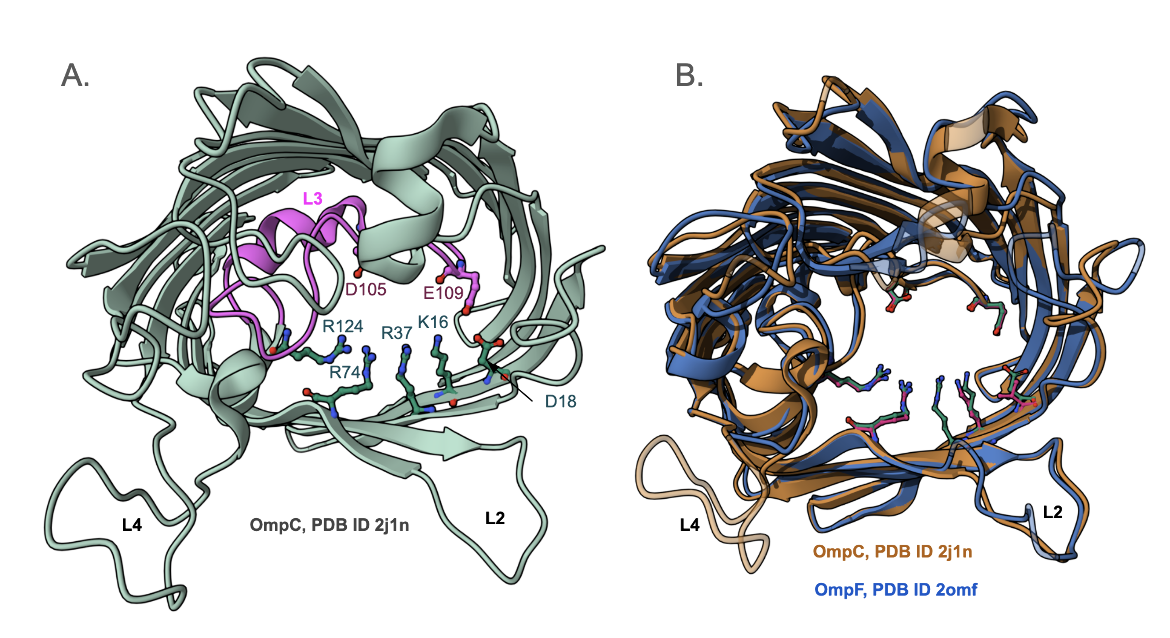
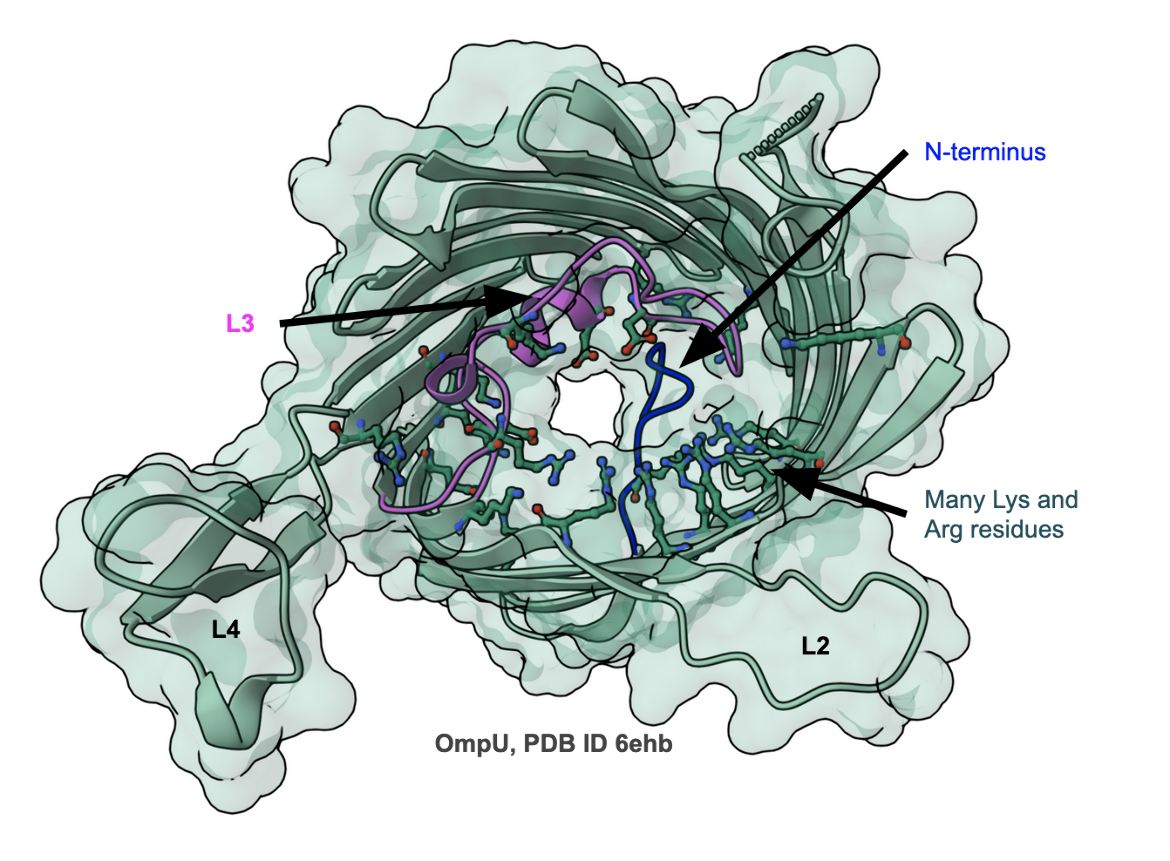
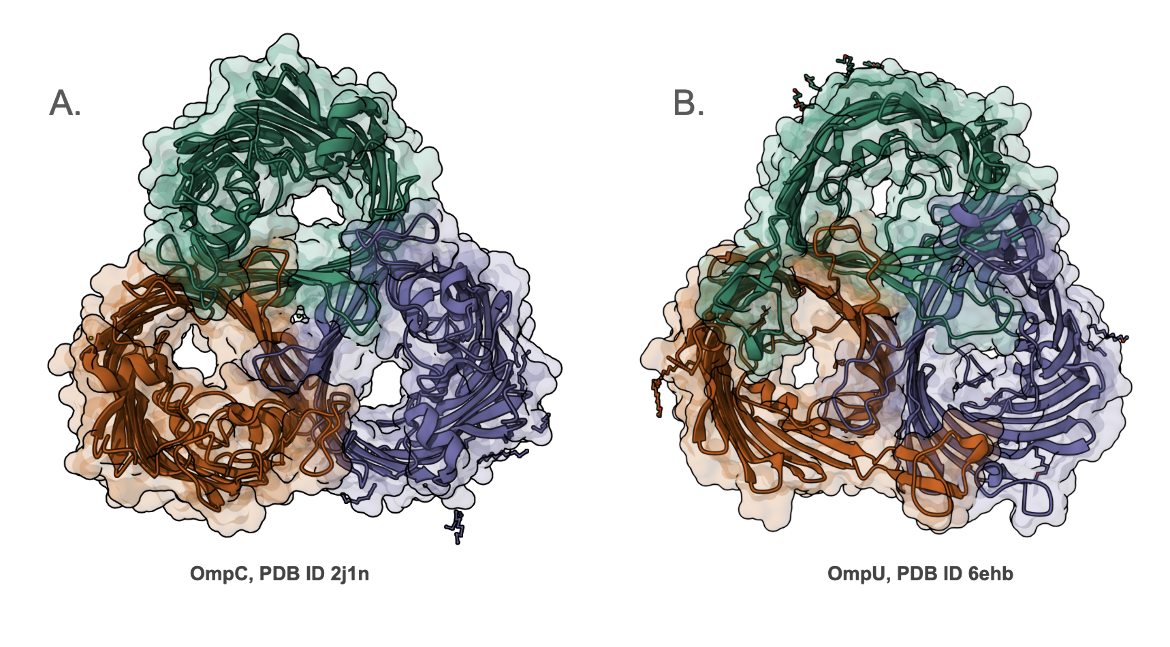
Most porins are homotrimeric in structure - i.e., there are three copies of the protein that interact with each other and are positioned across the outer membrane (Figure 1). They have variable selectivity, ranging from cation-selective, anion-selective, or selective for different compounds. The central pore in each of the images of the E. coli OmpC (Figure 1A) and V. cholera OmpU (Figure 1B) is the white space at the center of the β-barrels. Note that the pore in OmpC is larger than that in OmpU.
|
| Figure 1. Side-by-side comparison of the structures of outer membrane proteins - A. E. coli OmpC (PDB ID 2j1n, Basle et al., 2006), and B. V. cholera OmpU (PDB ID 6ehb, Pathania et al., 2018). |
OmpC
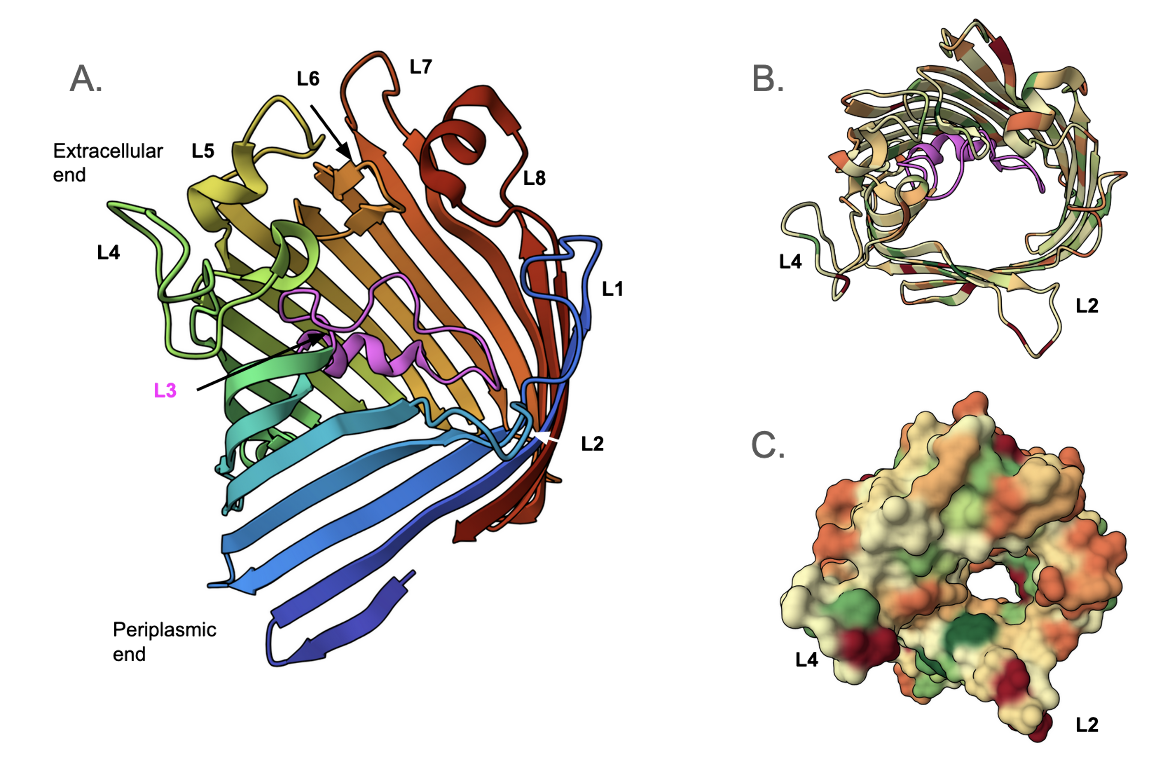
The E. coli OmpC was one of the first antibiotic-transporting porins identified in a gram-negative bacterium (Achouak et al., 2001). The 2.0 Å structure of osmoporin OmpC from strain K12 of E. coli (Baslé et al., 2006) was determined using the structure of porin OmpK36 from K. pneumoniae as the search model. The monomeric unit of OmpC (Figure 2A) consists of sixteen antiparallel β-strands that form a β-barrel structure with a central pore (Lou et al., 2011). These strands are connected by short turns at the periplasmic rim and long, irregular, extracellular loops (denoted L1, L2, L4-L8) (Baslé et al., 2006). A view down the pore of the monomeric porin shows that L3 forms a short helical structure (colored in magenta) and is positioned in the pore (Figure 2B). The surface view shows the distribution of hydrophobic (green) and hydrophilic (red) amino acid side chains in the porin (Figure 2C).
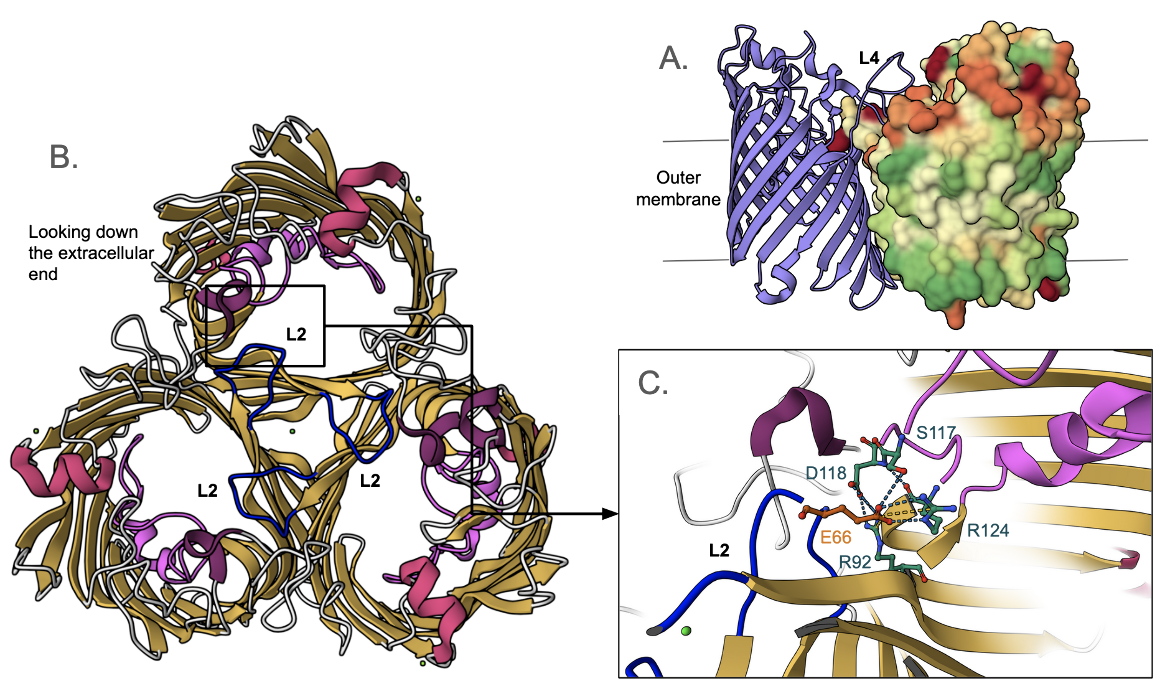
The OmpC homotrimer formation in E. coli is promoted by hydrophobic interactions between the monomeric barrel surfaces (Figure 3A). Loops L1, L2, L4-L8 are the extracellular structures that connect the β-strands of the monomeric unit to form a hollow β-barrel. Each of the three L2 loops, which are also known as the “latching loops”, reaches over into the adjacent monomer to hold the trimer together (Baslé et al., 2006). As the latching loop extends into the adjacent monomer, a glutamic acid residue on the loop (Glu66) forms a conserved salt bridge with one of the arginine residues (Arg124) inside the barrel of the monomer (Lou et al., 2011). Loop L4 is the extracellular connection that contributes to the stabilization of the homotrimer and forms the entrance to the pore of the protein (Lou et al., 2011). Loop L3 is not extracellular - it folds back into the pore and extends along the inside of the barrel wall (Baslé et al., 2006). This causes the pore to be narrowed about halfway through, resulting in the formation of a “constriction zone” or an “eyelet” (Lou et al., 2011).
Two acidic amino acid residues on loop L3, and one on the strand, face four basic amino acid residues on the inner barrel wall creating a strong transverse electrical field at the constriction zone (Figure 4A). Many of these critical amino acid residues are highly conserved (Figure 4B) in all OmpC porins and even in OmpF porins, which are the other major non-specific porins found in E. coli. Superimposing the structures of OmpC and OmpF shows that they have a root mean square deviation of 1Å, with a sequence identity of 64% over 325 amino acids. Thus, the transverse electrical field, found in all OmpC and OmpF porins, allows the pore to be permeable to hydrophilic molecules (Lou et al., 2011). It is even thought that zwitterionic antibiotics, which contain a negatively charged ion on one end and a positively charged ion at the other, interact with these acidic and basic amino acid residues at the entrance to the channel (Lou et al., 2011). This interaction between the antibiotic molecule and the charged amino acid residues facilitates the translocation of the polar molecule through the pore (Lou et al., 2011).
Learn more about ampicillin binding to OmpF.
OmpU
V. cholerae contains a variety of outer membrane proteins, of which OmpU and OmpT have been the most extensively studied (Pathania et al., 2018). Despite low (<20%) sequence identity, they are functional homologs of E. coli OmpF/C. While OmpU allows the transport of larger sugars than OmpT, it is less efficient in mediating the passage of β-lactam antibiotics. The structure of OmpU shows that its overall architecture is similar to that of OmpC/OmpF. However, the pore itself is constricted. Close examination of the structure reveals that the N-terminus of OmpU folds into the pore, constricting it (see blue loop in Figure 5). In addition, the pore is lined by a significantly higher number of Arg and Lys residues, changing the internal channel electrostatics. Biophysical studies of OmpU and OmpT show that bile salt can only pass through OmpT, but both interact with carbapenem antibiotics (Pathania et al., 2018).
Outer Membrane Proteins in Eukaryotes
It is important to note that outer membrane protein channels are found not only in gram-negative bacteria but within eukaryotic cells as well. The endosymbiotic theory, which explains that eukaryotic organelles such as chloroplasts and mitochondria were once free-living prokaryotes, is supported by the fact that these organelles are surrounded by multiple membranes, similar to gram-negative bacteria and other prokaryotes. Therefore, there must be a way through which organelles, such as mitochondria, exchange metabolites with the cytoplasm. The outer membrane of mitochondria consists of channels, known as voltage-dependent anion channels (VDACs) that are responsible for moving metabolites and ions between the cytoplasm and the inter-membrane space of mitochondria (Ujwal et al., 2008). In this discussion we focus on porins - since they function as channels for transporting antibiotics across the bacterial outer membrane.
Resistance Due to Decreased Permeability
Decreased influx of β-lactams into bacteria - either by reducing the expression of porins or impairing their function through mutations, can lead to resistance. When the antibiotic is no longer able to reach its intracellular target, the bacterial cell becomes less susceptible to that antimicrobial drug (Masi et al., 2013).
Resistance via absence
Most antibiotic-resistant strains of E. coli have been found to have reduced or no expression of the OmpF porin. Thus it appears that the influx of antibiotics is prominently governed by OmpF (Lou et al., 2011). Deletion of the porin gene or gene product results in such resistance.
Resistance due to mutations
In E. coli OmpC also contributes to the transport of antibiotics (Lou et al., 2011). In a patient who had a chronic E. coli infection of liver cysts, it was noted that the cells lacked OmpF expression so the OmpC protein served as the main pathway for antibiotic influx in the isolates (Lou et al., 2011). This OmpC protein was also found to have polypeptide sequence alterations. Although the variant OmpC formed the same trimeric structure as the functional porin, they failed to transport the antibiotic - leading to resistance.
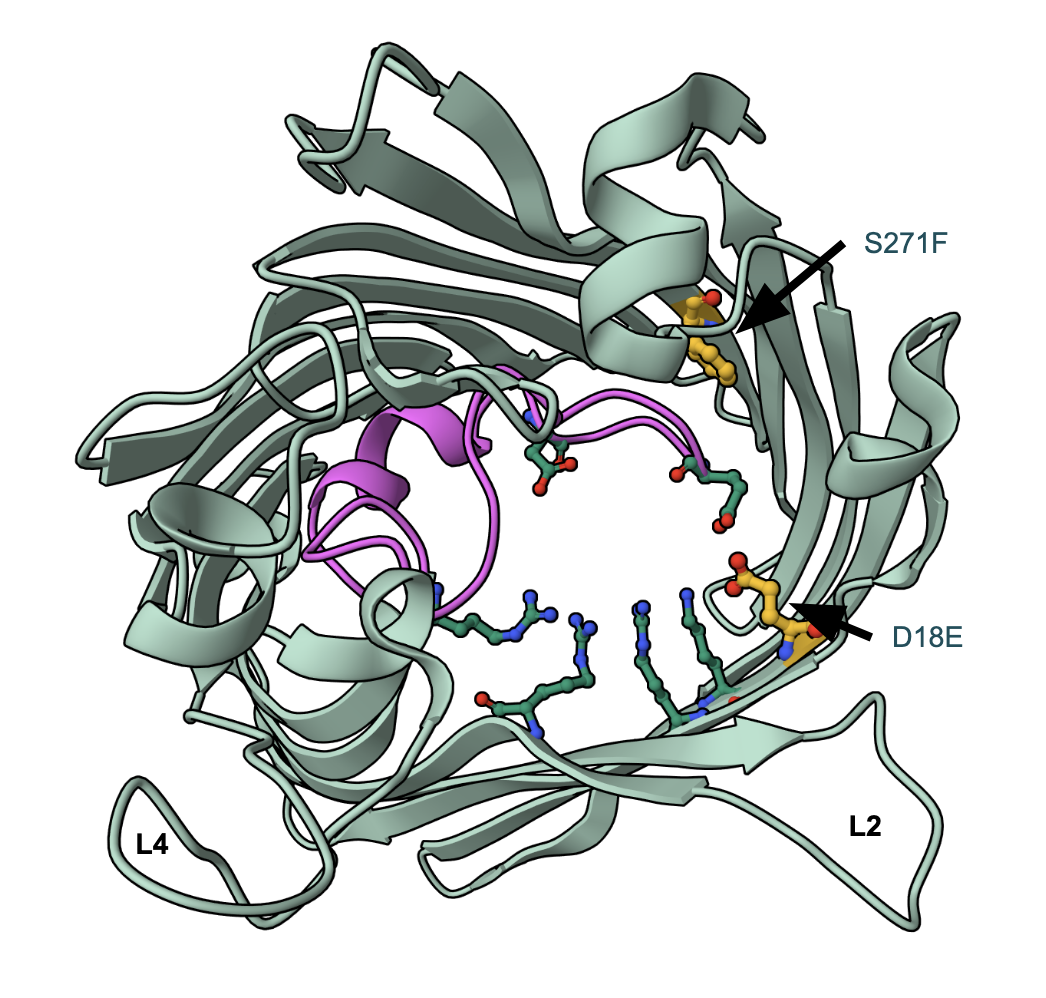
In one OmpC variant, the Asp18 of all three monomers had been replaced by Glu (Lou et al., 2011). Compared to Asp, Glu has a longer side chain, which now protrudes into the lumen of the pore and forms new interactions with surrounding residues (Lou et al., 2011). More specifically, the sidechain carboxyl group of Glu18 makes a new hydrogen bond with Lys16 and also with Tyr35. These interactions make the eye of the porin a tad bit smaller (Lou et al., 2011).
In another OmpC variant, in addition to the D18E substitution, when the Ser271 is replaced by Phe in all three monomeric units of the trimer (Figure 6), and the tip of loop L3 shifts slightly further out into the lumen of the pore (Lou et al., 2011). Although this does not seem to affect the eyelet of the pore, it causes a slight movement of loop L3, leading to resistance.
Replacement of Asp by Glu at position 18 resulted in a disruption of the original hydrogen bond network present in the transverse charge field at the constriction zone (Lou et al., 2011). Although the substitution does not appear to have a significant effect on the size of the pore eyelet, the channel electrostatics are altered (Lou et al., 2011). Perhaps this plays a role in making the porin less permeable to cefotaxime (Lou et al., 2011). These clinical findings suggest that the reduction of antibiotic influx, especially the uptake of larger polar molecules, can be attributed to changes in the transverse electrical field of the eyelet, which impair the function of OmpC (Lou et al., 2011).
Combatting Antibiotic Resistance by Porins
When the function of OmpC and other similar porins is impaired, the result is a membrane that is significantly less permeable to antibiotics and thus a bacterium that is less susceptible to the drug. There are some natural compounds, that behave as membrane permeabilizers (Bolla et al., 2011). For example, eugenol, a key component of clove oil derived from Eugenia aromatica (Bolla et al., 2011), disrupts the cytoplasmic membrane of gram-negative bacteria so that molecules, such as antimicrobial drugs, can penetrate the barrier (Bolla et al., 2011). When ten different antibiotics effective against gram-negative bacteria were each combined with eugenol, it was found that the minimum inhibitory concentration (MIC) of antibiotics decreased by 5-1000 fold (compared to when the antibiotic was used alone) (Bolla et al., 2011). This suggests that eugenol allows the outer membrane to become even more permeable to extracellular compounds. Thus the combination of eugenol and antibiotics is synergistic (Bolla et al., 2011). In addition, polymyxin B and polymyxin E can also increase bacterial susceptibility to antibiotics by acting on the outer membrane. However, these compounds can prove to be toxic to eukaryotic cells as well (Bolla et al., 2011).
References
Achouak, W., Heulin, T., Pagès, J. (2001). Multiple facets of bacterial porins. FEMS Microbiology Letters,199(1), 1-7. https://doi.org/10.1111/j.1574-6968.2001.tb10642.x
Basle, A., Storici, P., Rummel, G., Rosenbusch, J., Schirmer, T. (2006). Osmoporin OmpC. https://doi.org/10.2210/pdb2J1N/pdb PDB ID: 2J1N
Bolla, J., Alibert-Franco, S., Handzlik, J., Chevalier, J., Mahamoud, A., Boyer, G., Kieć-Kononowicz, K., Pagès, J. (2011). Strategies for bypassing the membrane barrier in multidrug resistant Gram-negative bacteria. FEBS Letters,585(11), 1682-1690. https://doi.org/10.1016/j.febslet.2011.04.054
Galdiero, S., Falanga, A., Cantisani, M., Tarallo, R., Pepa, M. E., Doriano, V., Galdiero, M. (2012). Microbe-Host Interactions: Structure and Role of Gram-Negative Bacterial Porins. Current Protein and Peptide Science,13(8), 843-854. https://doi.org/10.2174/138920312804871120
Gram-positive bacteria. (2018, April 10). In Wikipedia. Retrieved from https://en.wikipedia.org/wiki/Gram-positive_bacteria
Jia, B., Raphenya, A. R., Alcock, B., Waglechner, N., Guo, P., Tsang, K. K., Lago, B. A., Dave, B. M., Pereira, S., Sharma, A. N., Doshi, S., Courtot, M., Lo, R., Williams, L. E., Frye, J. G., Elsayegh, T., Sardar, D. Westman, E. L., Pawlowski, A. C., Johnson, T. A., Brinkman, F. S., Wright, G. D., McArthur, A. G. (2017) CARD 2017: expansion and model-centric curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Research 45, D566-573. https://doi.org/10.1093/nar/gkw1004
Lou, H., Chen, M., Black, S. S., Bushell, S. R., Ceccarelli, M., Mach, T., Beis, K., Low, A. S., Bamford, V. A., Booth, I. R., Bayley, H., Naismith, J. H. (2011). Altered Antibiotic Transport in OmpC Mutants Isolated from a Series of Clinical Strains of Multi-Drug Resistant E. coli. PLoS ONE,6(10). https://doi.org/10.1371/journal.pone.0025825 PDB IDS: 2XE3, 2XE5
Masi, M. (2013). Structure, Function and Regulation of Outer Membrane Proteins Involved in Drug Transport in Enterobactericeae: The OmpF/C – TolC Case. The Open Microbiology Journal,7(1), 22-33. https://doi.org/10.2174/1874285801307010022
Nakae, T. (1976) Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 71(3):877-84. https://doi.org/10.1016/0006-291x(76)90913-x
Nikaido, H. (1985) Role of permeability barriers in resistance to beta-lactam antibiotics. Pharmacol Ther. 27(2):197-231. https://doi.org/10.1016/0163-7258(85)90069-5
Pagès, J. M., James, C., Winterhalter, M. (2008) The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol 6, 893–903. https://doi.org/10.1038/nrmicro1994
Pathania, M., Acosta-Gutierrez, S., Bhamidimarri, S. P., Baslé, A., Winterhalter, M., Ceccarelli, M., van den Berg, B. (2018) Unusual Constriction Zones in the Major Porins OmpU and OmpT from Vibrio cholerae. Structure. 26(5):708-721.e4. https://doi.org/10.1016/j.str.2018.03.010
Ujwal, R., Cascio, D., Colletier, J., Faham, S., Zhang, J., Toro, L., Ping, P., Abramson, J. (2008). The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proceedings of the National Academy of Sciences,105(46), 17742-17747. https://doi.org/10.1073/pnas.0809634105. PDB ID: 3EMN
April 2025, Gauri Patel and Shuchismita Dutta; Reviewed by Dr. Ann Stock
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/amr-mech/porins