Cell Wall Biosynthesis
Cells are the fundamental unit of life for all living organisms. From small, unicellular organisms to complex, multicellular organisms, an important feature of cells is the semipermeable cell membrane. In most cells, the cell membrane consists of a phospholipid bilayer with hydrophilic exterior heads (i.e., the intra- and extracellular faces) and a hydrophobic interior region. Proteins embedded in the cell membrane regulate what goes in and out of the cell, helping to maintain homeostasis – a stable state for an organism's internal environment. Consequently, the cell membrane functions as a basic line of defense against environmental factors and pathogens. For many prokaryotes, including bacteria, the cell membrane alone is not enough to ensure survival. Environmental conditions and bacterial viruses have forced bacteria to evolve and develop an additional feature outside the cell membrane: the peptidoglycan cell wall.
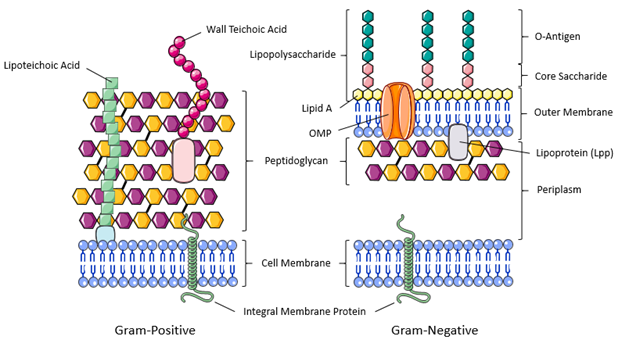
The Cell Wall
A cell wall is a structural layer that surrounds the entire cell and serves as a protective barrier. Like the cell membrane, the cell wall can selectively allow passage of nutrients and waste materials to preserve homeostasis (Silhavy et al., 2010). Beyond these similarities, the bacterial cell wall provides additional functions. The cell wall is durable and maintains cell shape, a critical characteristic in many pathways and mechanisms such as motility. Without a cell wall, many bacterial cells would lyse or break open from internal turgor pressure or osmotic shock as the cells move from one environment to another. The cell wall also displays the necessary characteristics of being dynamic in specific stages of the cell cycle, in particular during cell division and reproduction.
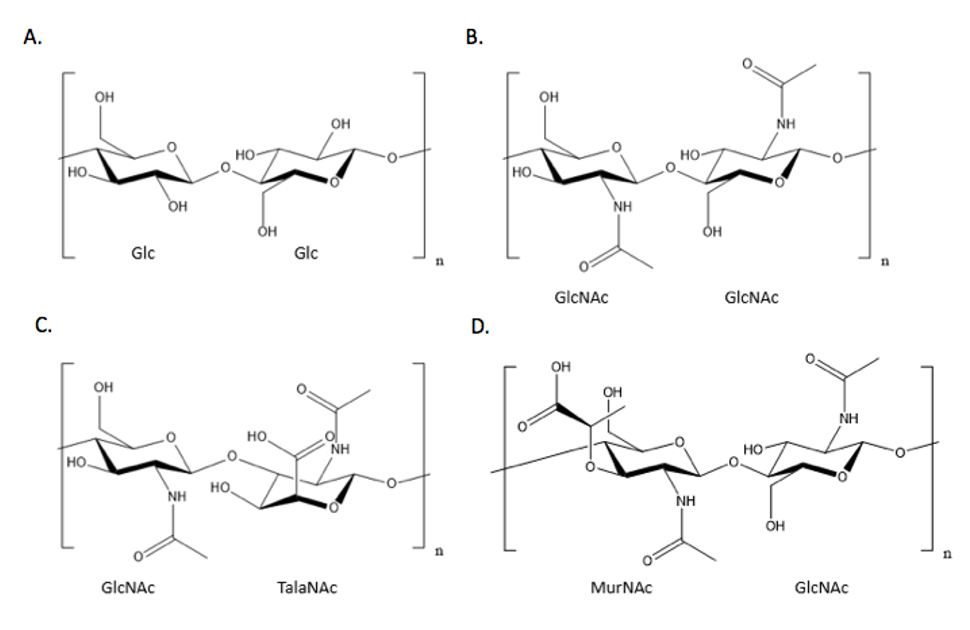
Cell walls can be found in many diverse organisms – from the cells of unicellular organisms such as archaebacteria and eubacteria to the cells of eukaryotes such as fungi, plants, and certain protists. The defining characteristic of a cell wall at the molecular level is the presence of long-chain molecules known as polymers, which feature the repetition of small organic compounds – typically glucose derivatives.
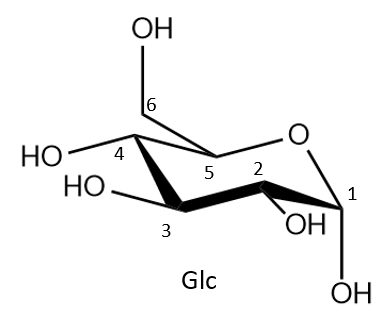
- The plant cell wall consists primarily of cellulose, which is a long polymer chain with repeats of linked D-glucose units (Figure 1). Other contributing polysaccharides involved in the composition of the cell wall are pectin and hemicellulose, which form matrices contributing to the rigidity of the plant cell wall (Keegstra, 2010) (see Figure 2A).
- The fungal cell wall primarily consists of chitin, a polymer containing linked N-acetylglucosamine units. Fungal cell walls also feature glucans, polymers of linked glucose monomers, and glycoproteins. Chitin, glucans, and glycoproteins are covalently cross-linked to each other to ensure the integrity of the cell wall (Bowman and Free, 2006) (see Figure 2B).
- In bacteria, the cell wall consists of a different polymer called peptidoglycan, named to reflect the integration of both amino acid peptides (peptido-) and sugars (-glycan) (Figure 2D). The structure of peptidoglycan is very similar to that of pseudopeptidoglycan found in archaea, where both possess GlcNAc and differ in the secondary glucose derivative subunit (see Figure 2C).
Peptidoglycan: Integral Polymer of Bacterial Cell Walls
Peptidoglycan, also known as murein, is a polymer consisting of disaccharide units composed of two distinct glucose derivatives N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc). The GlcNAc and MurNAc alternate to form long chains of peptidoglycan that are cross-linked by short, peptidyl bridges attached at MurNAc subunits.
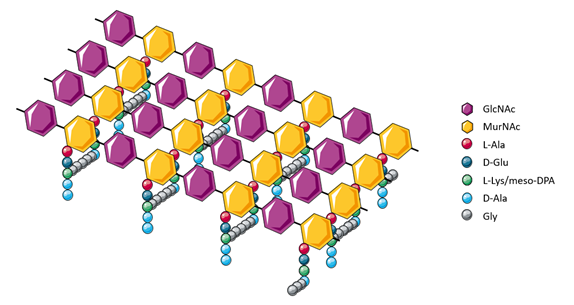
The short polypeptides that form the chemical cross-links or bridges are typically composed of four different amino acids organized into linear pentapeptides. Although the composition of these pentapeptides varies amongst different species of bacteria, the most common sequence is L-alanine, D-glutamine, L-lysine or meso-diaminopimelic acid (DPA), and two consecutive D-alanine residues (Lovering et al., 2012, Walsh, 1989). The stem peptides are directly cross-linked to each other between the D-alanine of one peptide and the meso-DPA or L-lysine of another peptide, usually a cleaved tetrapeptide in which the additional D-alanine has been removed. The cross-linking of peptidoglycan polymers results in a lattice-like mesh, which allows peptidoglycan to stabilize the shape of the cell and function as a protective barrier.
|
| Figure 3. Cross-linking between peptidoglycan polymers creates a robust mesh structure. |
It is remarkable that two D-isomeric amino acids (D-alanine and D-glutamine) are integral to the structure of the peptidoglycan cell wall. In living organisms, amino acids exhibit the property of homochirality, where almost all observed amino acids found adopt the L-isomeric form. For example, a recent analysis of Swiss-Prot revealed only 837 D-isomeric amino acids out of the 187 million analyzed amino acids (Khoury et al., 2011). The evolutionary advantage of D-isomeric amino acids is thought to provide protection of the cell wall from proteases - since enzymes that break down proteins and peptides generally only recognize L-isomeric amino acids.
Cell Wall Synthesis
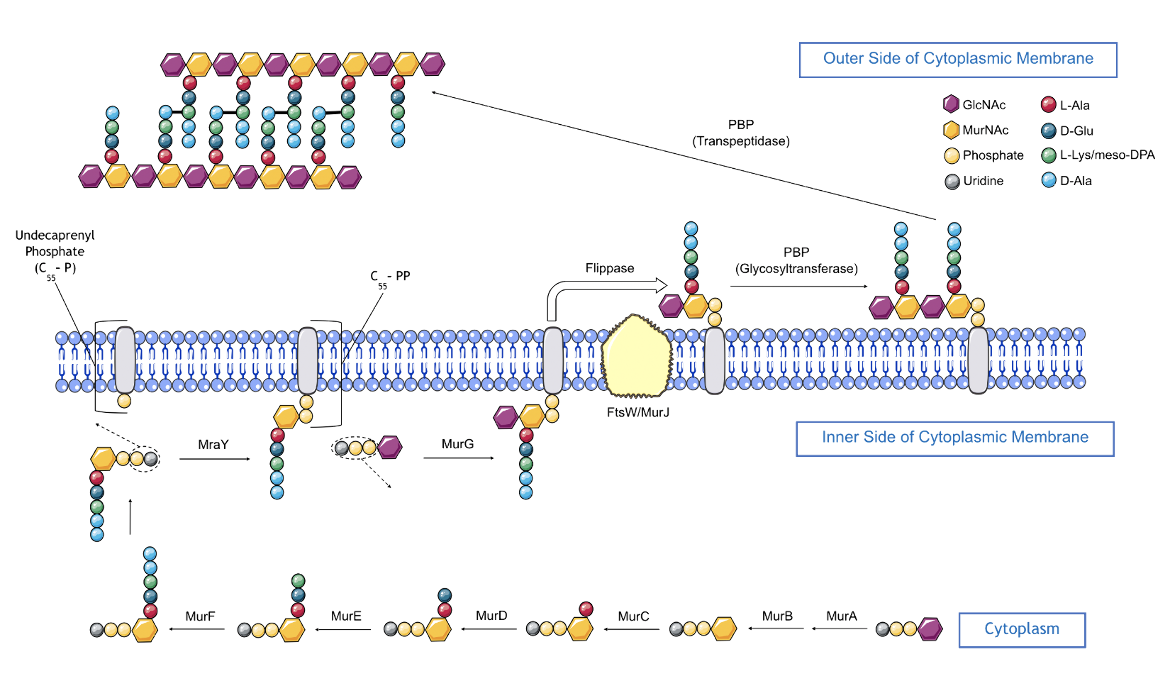
The process of cell wall peptidoglycan synthesis is complex, involving more than 20 different enzymes and proteins, which are responsible for making precursor molecules and extending the peptidoglycan layer (Typas et al., 2012). A schematic summary is provided below in Figure 4.
Peptidoglycan biosynthesis (depicted in Figure 4) starts inside the cell and involves a series of enzyme-catalyzed steps within the cytoplasm and inner leaflet of the cell membrane.
- MurA, a transferase enzyme, catalyzes the transfer of enolpyruvate from phosphoenolpyruvate (PEP) to UDP-GlcNAc, yielding enolpyruvyl UDP-GlcNAc.
- MurB then catalyzes the conversion of enolpyruvl UDP-GlcNAc to UDP-MurNAc (using NADPH cofactor).
- MurC catalyzes the ligation of the first amino acid, L-Ala, to UDP-MurNAc generating UDP-MurNAc-L-Ala (using ATP cofactor)
- MurD catalyzes the ligation of D-Glu to UDP-MurNAc-L-Ala, forming UDP-MurNAc-L-Ala-D-Glu (using ATP cofactor).
- MurE catalyzes the addition of either meso-DPA (in most gram-negative bacteria) or L-Lys (in most gram-positive bacteria) to UDP-MurNAc-L-Ala-D-Glu forming a UDP-MurNAc-tripeptide.
- Alanine Racemase catalyzes the conversion of L-Alanine (naturally abundant amino acid) to D-Alanine, creating building blocks required for cell wall biosynthesis.
- D-alanine-D-alanine ligase is an enzyme that links two D-Alanine residues to create the D-Ala-D-Ala dipeptide.
- MurF completes the formation of a pentapeptide by catalyzing the addition of a D-Ala-D-Ala dipeptide to UDP-MurNAc-tripeptide forming a UDP-MurNAc-pentapeptide (using ATP cofactor).
- MraY catalyzes the transfer of the UDP-MurNAc-pentapeptide to an undecaprenyl phosphate transport lipid on the inner leaflet of the phospholipid bilayer cell membrane forming lipid I and UMP.
- MurG catalyzes the transfer of GlcNAc from UDP-GlcNAc to MurNAc in lipid I, which yields lipid II. This is the final intracellular step in peptidoglycan cell wall synthesis.
Completion of peptidoglycan cell wall synthesis next requires translocation of lipid II across the cell membrane from the cytoplasm. This step is known to be protein-mediated, but the exact protein responsible for this “flippase” activity is a matter of debate (Ruiz, 2015). Current evidence suggests several candidates, namely AmJ, FtsW, and MurJ, and recent studies have proposed models involving the combined activities of several candidates.
Once lipid II has been translocated to the outer leaflet of the phospholipid bilayer cell membrane, it undergoes polymerization and cross-linking reactions catalyzed by monofunctional or bifunctional enzymes, also known as penicillin-binding proteins or PBPs. Polymerization of lipid II begins with PBP glycosyltransferase-catalyzed linking lipid II glycans - the GlcNAc moiety from one lipid II unit and the MurNAc moiety from the other lipid II unit are linked. This process continues with multiple elongation steps involving the joining of additional lipid II units to form a nascent glycan strand. Proteins, such as UppP (Undecaprenyl Pyrophosphate Phosphatase), dephosphorylates the undecaprenyl pyrophosphate to recycle and regenerate undecaprenyl phosphate for the synthesis of lipid I units.
Then, 4-3 cross-linking of the nascent peptidoglycan strands to one another is catalyzed by PBP transpeptidases (also known as D,D transpeptidases), which join the fourth amino acid (D-Ala) of the donor pentapeptide unit to the third amino acid (meso-DPA in gram-negative bacteria; L-Lys in gram-positive bacteria) of the acceptor pentapeptide, and this results in the release of the terminal amino acid (D-Ala) from the donor.
Although these 4-3 cross-links are predominant in bacteria, 3-3 cross-links have also been reported in the peptidoglycan of several bacterial species, such as Escherichia coli and Mycobacterium tuberculosis. These 3-3 cross-links are formed between the third amino acid (meso-DPA) in both the strands. The enzymes responsible for this cross-linking activity are known as L,D-transpeptidases (Kumar et al., 2012).
|
| Figure 5. A schematic summary of the peptidoglycan biosynthesis pathway. The third amino acid of the pentapeptide is L-Lys in most gram-positive bacteria and meso-DPA in most gram-negative bacteria. |
Cell Wall Synthesis as a Target of Antibiotics
The role of a peptidoglycan cell wall in the survival of bacteria is well established. Without a cell wall, many bacteria are vulnerable to the surrounding environment, making them susceptible to lysis and death. Cell wall synthesis can be interrupted by targeting enzymes both inside and outside the phospholipid bilayer cell membrane. Approved antibiotics that inhibit intracellular enzymes are chemically diverse, reflecting the complexity of the chemistry of cell wall synthesis. In contrast, approved antibiotics that inhibit extracellular enzymes are much less diverse because they focus on just two chemical features of the peptidoglycan cell wall.
Targeting extracellular enzymes has proven to be a successful antibiotic approach for two principal reasons. First, the discovery of natural products, such as penicillin G, provided medicinal chemists with roadmaps for synthesizing variants with desired properties. Second, the required chemical-physical attributes of drugs that target extracellular enzymes are less stringent than those targeting proteins inside the bacterial cell, because they do not have to pass through the phospholipid bilayer cell membrane.
Table 1: Antibiotics that target cell wall synthesis and their enzymatic targets. Note that many of the compounds listed in this table may inhibit the enzyme mentioned but do not have any antibacterial activity.
| Target | Antibiotic/Inhibitor | Comments |
|---|---|---|
| MurA | Fosfomycin | US FDA approved for clinical use |
| MurB | 4-Thiazolidinone derivatives | no antibacterial activity |
| MurC | Feglymycin | inhibits both MurA and MurC in vitro but has no antibacterial activity |
| MurD | Sulfonamide derivatives | no antibacterial activity (Simčič et al., 2012) |
| MurE | Phosphinate | no antibacterial activity (Simčič et al., 2012) |
| MurF | Diarylquinolines | affect cell wall synthesis (Baum et al., 2009) |
| MraY | Tunicamycin | inhibits the bacterial enzyme but also mammalian enzymes. There are other MraY inhibitors that are more specific to bacteria, but not registered by the FDA (Dini, 2005) |
| MurG | Murgocil | staphylococcal-specific inhibitor of the enzyme, antibacterial, but not registered by FDA (Mann et al., 2013) |
| Flippase (MurJ) | Humimycin | anthranillic acid derivative; antibacterials but not used as drugs (Chu et al., 2016) |
| PBP Glycosyltransferase | Moenomycin; | enzyme inhibitor, used in animal health, but not for treating human infections (Otash and Walker, 2010) |
| PBP Transpeptidase | Beta-Lactams | US FDA approved for clinical use |
| PBP Transpeptidase | Vancomycin | Binds to the transpeptidase substrate; US FDA approved for clinical use |
| D-Ala-D-Ala Ligase, Alanine racemase | D-cycloserine | US FDA approved for clinical use |
| Lipid I and Lipid II | Ramoplanin | binds to inhibit both MurG and transglycosylase (Lo et al., 2000) |
References
Baum, E. Z., Crespo-Carbone, S. M., Foleno, B. D., Simon, L. D., Guillemont, J., Macielag, M., Bush, K. (2009) MurF inhibitors with antibacterial activity: effect on muropeptide levels. Antimicrob Agents Chemother. 53(8):3240-7. https://doi.org/10.1128/aac.00166-09
Bowman, S. M., and Free, S. J. (2006) The structure and synthesis of the fungal cell wall. Bioessays 28, 799-808. https://doi.org/10.1002/bies.20441
Chu, J., Vila-Farres, X., Inoyama, D., Ternei, M., Cohen, L. J., Gordon, E. A., Reddy, B. V., Charlop-Powers, Z., Zebroski, H. A., Gallardo-Macias, R., Jaskowski, M., Satish, S., Park, S., Perlin, D. S., Freundlich, J. S., Brady, S. F. (2016) Discovery of MRSA active antibiotics using primary sequence from the human microbiome. Nat Chem Biol. 12(12):1004-1006. https://doi.org/10.1038/nchembio.2207
Dini, C. (2005) MraY Inhibitors as Novel Antibacterial Agents. Curr Top Med Chem. 5(13):1221-36. https://doi.org/10.2174/156802605774463042
Keegstra, K. (2010) Plant cell walls. Plant Physiology 154, 483-486. https://doi.org/10.1104/pp.110.161240
Khoury, G. A., Baliban, R. C., and Floudas, C. A. (2011) Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Scientific Reports 1, 90. https://doi.org/10.1038/srep00090
Kumar, P., Arora, K., Lloyd, J. R., Lee, Y., Nair, V., Fischer, E., Boshoff H. I. M., Barry, C. E. (2012). Meropenem inhibits D,D-carboxypeptidase activity in Mycobacterium tuberculo-sis. Molecular Microbiology, 86, 367-381. https://doi.org/10.1111/j.1365-2958.2012.08199.x
Lo, M-. C., Men, H., Branstrom, A., Helm, J., Yao, N., Goldman, R., Walker, S., (2000) J. Am. Chem. Soc. 122, 14, 3540–3541. https://doi.org/10.1021/ja000182x
Lovering, A. L., Safadi, S. S., and Strynadka N. C. J. (2012) Structural perspective of peptidogly-can biosynthesis and assembly. Annual Review of Biochemistry 81, 451-478. https://doi.org/10.1146/annurev-biochem-061809-112742
Mann, P. A., Müller, A., Xiao, L., Pereira, P. M., Yang, C., Ho Lee, S., Wang, H., Trzeciak, J., Schneeweis, J., Dos Santos, M. M., Murgolo, N., She, X., Gill, C., Balibar, C. J., Labroli, M., Su, J., Flattery, A., Sherborne, B., Maier, R., Tan, C. M., Black, T., Onder, K., Kargman, S., Monsma, F. J. Jr., Pinho, M. G., Schneider, T., Roemer, T. (2013) Murgocil is a highly bioactive staphylococcal-specific inhibitor of the peptidoglycan glycosyltransferase enzyme MurG. ACS Chem Biol. 8(11):2442-51. https://doi.org/10.1021/cb400487f
Ostash, B., Walker, S. (2010) Moenomycin family antibiotics: chemical synthesis, biosynthesis, and biological activity. Nat Prod Rep. 27(11):1594-617. https://doi.org/10.1039/c001461n
Ruiz, N. (2015) Lipid Flippases for Bacterial Peptidoglycan Biosynthesis. Lipid Insights 8, 21-31. https://doi.org/10.4137/LPI.S31783
Silhavy, T. J., Kahne, D., and Walker, S. (2010) The bacterial cell envelope. Cold Spring Harbor Perspectives in Biology, 2:a000414. https://doi.org/10.1101/cshperspect.a000414
Simčič, M., Sosič, I., Hodošček, M., Barreteau, H., Blanot, D., Gobec, S., Grdadolnik, S. G. (2012) The binding mode of second-generation sulfonamide inhibitors of MurD: clues for rational design of potent MurD inhibitors. PLoS One. 7(12):e52817. https://doi.org/10.1371/journal.pone.0052817
Typas, A., Banzhaf, M., Gross, C. A., and Vollmer, W. (2012) From the regulation of peptidogly-can synthesis to bacterial growth and morphology. Nature Reviews Microbiology 10, 123-136. https://doi.org/10.1038/nrmicro2677
Walsh, C. T. (1989), Enzymes in the D-alanine branch of bacterial cell wall peptidoglycan assembly. J Biol Chem., 264, 2393-6. https://www.jbc.org/article/S0021-9258(19)81624-1/pdf
March 2025, Gauri Patel, Helen Gao, Shuchismita Dutta: Reviewed by Dr. Lynn Silver
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/cellwall-biosynth