Rifamycin
Discovery
Rifamycins were isolated by scientists in Milan as a mixture of the natural metabolites of Nocardia mediterranei (Sensi, 1983). P Sensi and his colleagues had the habit of giving new compounds unique nicknames. They called these molecules rifamycins, after the title of the French gangster film, Rififi, directed by Jules Dassin (Aronson 1999). The first compound purified from this mixture was rifamycin B, a molecule that had limited antibiotic activity. A chemically modified version of the same (called rifamycin SV) was found to be more potent. Systematic structural modifications of the functional groups of the rifamycin molecule led to the finding of a molecule that was active when administered orally (Sensi, 1983). A series of rifamycin analogs were produced semi-synthetically, leading to the creation of rifampin, rifapemntine, rifabutin, and rifamixin (Floss and Yu, 2005).
As a class, the rifamycins display a broad spectrum of antibiotic activity against gram-positive and, to a lesser extent, gram-negative bacteria (Campbell et al., 2018; Ho et al., 2018). They exhibit sterilizing activity against Mycobacterium tyberculosis and are first-line drugs for the treatment of tuberculosis (Floss and Yu, 2005).
Overview of Chemistry
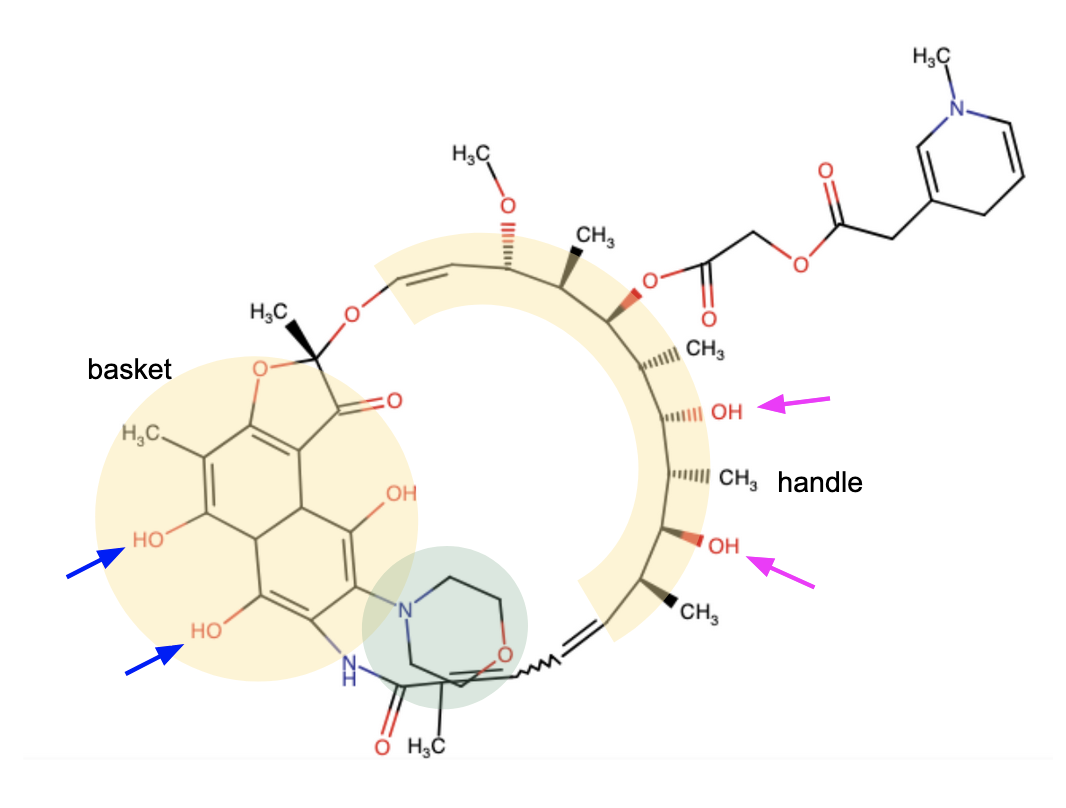
Rifamycins belong to the family of ansamycin antibiotics. These molecules have a basket-like architecture (Figure 1) - where an aromatic moiety resembles the basket structure, while the aliphatic chain that bridges across it resembles the handle, which is called ansa in Latin. The core structural features required for antibiotic activity are two hydroxyls at C 21 and C 23 positions of the handle region (marked with magenta arrows) and two polar groups at C 1 and C 8 positions of the aromatic moiety (marked with blue arrows). The position 3 in the aromatic nucleus is commonly derivatized in different members of the family.
Floss and co-workers discovered the first rifamycin biosynthetic gene cluster comprised of 34 genes in the bacterium Amycolatopsis mediterranei S699 (August et al., 1998). The ansa chain of rifamycin is biosynthesized from propionate and acetate units by a multifunctional type I polyketide synthase. Since rifamycin production by this organism is orchestrated by a single gene, careful study of this cluster's structural, resistance, export, and regulatory genes could yield new antibiotics that may be effective against rifamycin-resistant
microorganisms (August et al., 1998).
Resistance
Since pathogens develop resistance to this antibiotic at a high rate (Floss and Wu, 2005), rifamycins are always prescribed as a part of drug combinations for the treatment of systemic infections. The predominant mechanism of resistance to rifamycins is drug target (RNA polymerase β-subunit, RpoB) modification by mutation. The antibiotic itself may also be chemically modified and inactivated. Learn more about rifampicin resistance.
References
August, P. R., Tang, L., Yoon, Y. J., Ning, S., Müller, R., Yu, T. W., Taylor, M., Hoffmann, D., Kim, C. G., Zhang, X., Hutchinson, C. R., Floss, H. G. (1998) Biosynthesis of the ansamycin antibiotic rifamycin: deductions from the molecular analysis of the rif biosynthetic gene cluster of Amycolatopsis mediterranei S699. Chem Biol. 5(2):69-79. https://doi.org/10.1016/s1074-5521(98)90141-7
Campbell, E. A., Korzheva, N., Mustaev, A., Murakami, K., Nair, S., Goldfarb, A., Darst, S. A. (2001) Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell. 104(6):901-12. https://doi.org/10.1016/s0092-8674(01)00286-0
Floss, H. G., Yu, T. W. (2005) Rifamycin-mode of action, resistance, and biosynthesis. Chem Rev. 105(2):621-32. https://doi.org/10.1021/cr030112j
Ho, M., Hudson, B., Das, K., Arnold, E., and Ebright, R. (2009) Structures of RNA polymerase-antibiotic complexes. Curr. Opin. Structl. Biol. 19, 715-723. https://doi.org/10.1016/j.sbi.2009.10.010
Sensi, P. (1983) History of the development of rifampin. Rev Infect Dis. 5 Suppl 3:S402-6. https://doi.org/10.1093/clinids/5.supplement_3.s402
March 2025, Shuchismita Dutta; Reviewed by Dr. Richard Ebright
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/rna-synth/rp/rifamycin