Tirzepatide
Research on naturally occurring incretins isolated from some fish (e.g., lamprey, dogfish and paddlefish) has shown that they can act as dual agonist of the GLP-1, GIP, and glucagon receptors, to lower blood glucose levels and induce weight loss (reviewed in Conlon et al., 2022). Inspired by this idea long acting dual agonists were developed to treat type 2 diabetes and several obesity related health issues.
Tirzepatide is the first antidiabetic drug that is a dual agonist of both glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors. Developed by Eli Lilly, Tirzepatide was approved by the US FDA under the brand name Mounjaro, as a ‘first-in-class’ drug (Chavda et al., 2022).
Table 1. Basic Profile of Tirzepatide
| Description | injectable anti-diabetic drug |
| Target(s) | dual glucose-dependent insulinotropic polypeptide receptor (GIPR) and glucagon-like peptide-1 receptor (GLP-1R) |
| Generic Name | Tirzepatide |
| Commercial Name | Mounjaro |
| Combination Drug(s) | N/A |
| Other Synonyms | LY3298176 |
| IUPAC Name | Tirzepatide is a synthetic peptide (see the amino acid sequence below) |
| 3D Structure of Exenatide bound to target protein receptor GLP-1R | Chain P in PDB IDs 7rgp and 7rbt |
|
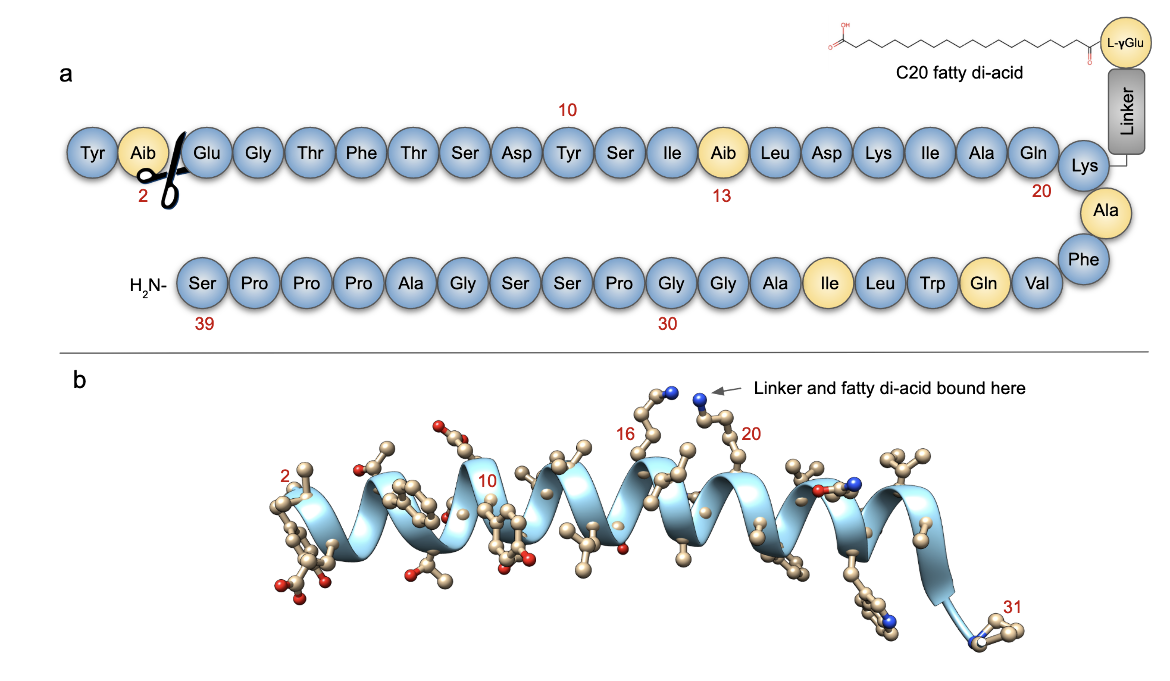
| Figure 1. 2D and 3D structures of Tirzepatide. a. The sequence of Tirzepatide (PubChem) has 39 amino acids. The schematic is based on information presented in Knerr et al., 2020. Amino acid that are either modified or different from both GLP-1 or GIP are highlighted in yellow. The DPP4 cleave site is indicated with the scissors. b. 3D structure of Tirzepatide (PDB ID 7rgp, Sun et al., 2022). Note: The C20 fatty diacid was disordered in the 3D structure, so is not shown here. The receptor and G-protein chains are hidden here for clarity. Click here to view the full structures interactively. |
Drug Information
Table 2. Chemical and physical properties of Tirzepatide
| Chemical Formula | C225H348N48O68 |
| Molecular Weight | 4810.52 Da |
| Calculated Predicted Partition Coefficient: cLogP | Not available |
| Calculated Predicted Aqueous Solubility: cLogS | Not available |
| Predicted Topological Polar Surface Area (TPSA) | Not available |
Drug Target
Tirzepatide is a dual agonist for both GLP-1 and GIP receptors. Learn more about GLP-1 receptors and GIP receptors.
Tirzepatide was developed as an oral or an injectable drug product. The addition of the C-20 fatty acid moiety to Lys20 is hypothesized to increase the binding dose-response ratio due to an increased albumin affinity. The replacement of Alanine 2 and 13 with Aib did not significantly change the binding affinity, but did result in a decline in potency as a substrate for DPP4. Collectively, these modifications prolong the half-life of Tirzepatide to approximately 5 days (FDA package insert). The mechanism of action is mediated by its binding to the GLP-1 receptor on β-cells of the pancreas to stimulate insulin secretion, slow gastric emptying, and reduce postprandial glucagon secretion in a glucose-dependent fashion. In addition, Tirzepatide also binds to GIP receptors on β-cells to co-stimulate insulin secretion by upregulating intracellular cAMP levels via GPCR-signaling.
Drug-Target Complex
Belonging to the class B G-protein-coupled receptors, GLP-1R (Figure 2) and GIPR (Figure 3) are both seven-transmembrane protein receptors. They both consisting of:
- An extracellular N-terminus that binds the C-terminal part of the incretin analog
- A seven transmembrane helical domain (TMD) with the a-helices separated by three intracellular loops and three extracellular loops
- An intracellular C-terminus that is responsible for signaling.
The EM structure of Tirzepatide bound to the complete GLP-1R and G-proteins (Figure 2, PDB ID 7rgp , Sun et al., 2022) shows some hydrophobic and some specific polar interactions between the peptidic drug and the GLP-1 receptor molecule. At the N-terminus, the Tyr1 of Tirzepatide forms weak hydrogen bonds with the side chain of Gln234. The Glu3 forms an ionic bond with Arg190 and a network of hydrogen bonds with Tyr152 and Tyr148 of the receptor (not shown here for clarity). These two Tyr residues in turn form pi-pi interactions. The Thr 7 forms a hyrogen bond with Lys197, while Asp9 forms a hydrogen bond with Arg380. The Ser8 and Ser11 of Tirzepatide form a network of hydrogen bonds involving the receptor residue Thr298. The Gln19 of Tirzepatide forms a hydrogen bond network with Thr35 and Val30 of the receptor, while the backbone atoms of Ile27 forms a hydrogen bond with Tyr69. Several of these interactions are similar to those seen in the binding of GLP-1 with GLP-1R. The C-terminal eight amino acids of the Tirzepatide are not visible in the structure so are not shown here.

|
| Figure 2. EM structure of GLP-1R (red) with bound Tirzepatide (gold) (PDB ID 7rgp, Sun et al., 2022). Location of the membrane, and G-proteins associated with the receptor are also shown. The insets show a close up of specific polar interactions between the Tirzepatide and the GLP-1R. |
The EM structure of Tirzepatide bound to the complete GIPR and G-proteins (Figure 3, PDB ID 7rbt, Sun et al., 2022) shows some hydrophobic and some specific polar interactions between the peptidic drug and the GIP receptor molecule. At the N-terminus, the Tyr1 of Tirzepatide makes Van der Waals contact with the receptor residues, the Glu3 forms an ionic bond with Arg183 and a network of hydrogen bonds with Tyr145 and Ser381 of the receptor. The Asp9 forms a hydrogen bond with Arg370, while Ser8 and Ser11 of Tirzepatide form a network of hydrogen bonds involving the receptor residue Glu288, Arg 289, and Asn290. The Asp15 of Tirzepatide forms a hydrogen bond with Ala32 of the receptor, the backbone atoms of Ile27 forms a hydrogen bond with Arg113 of the receptor, and the side chain of Tirzepatide’s Ser32 forms a hydrogen bond with Tyr200 of the GIPR. Several of these interactions are similar to those seen in the binding of GLP-1 with GLP-1R. The C-terminal seven amino acids of the Tirzepatide are not visible in the structure so are not shown here.

|
| Figure 3. EM structure of GIPR (red) with bound Tirzepatide (gold) (PDB ID 7rbt, Sun et al., 2022). Location of the membrane, and G-proteins associated with the receptor are also shown. The insets show a close up of specific polar interactions between the Tirzepatide and the GIPR. |
A comparison of the two complexes (Tirzepatide bound to GLP-1R vs GIPR) shows that several of the same amino acids of the peptide interact with corresponding amino acids in the GLP-1 with GLP-1R. Although the overall interactions look similar there are a few differences in the strengths of the interactions. Also the location of the C-20 fatty acid could not be discerned in these structures so their interactions are not discussed here.
Pharmacologic Properties
Table 3. Pharmacokinetics: ADMET of Tirzepatide
| Features | Comment(s) | Source |
| Bioavailability (%) | 80% following subcutaneous injection | DrugBank |
| IC50 (nM) | N/A | N/A |
| Ki (nM) | GIPR Ki = 0.135, SEM = 0.020 nM; GLP-1R Ki = 4.23, SEM = 0.23 nM | Nowak et al., 2022 |
| Half-life (hrs) | 120 hrs (~5 days) | DrugBank |
| Duration of Action | N/A | N/A |
| Absorption | N/A | N/A |
| Transporter(s) | Albumin | DrugBank |
| Metabolism | Tirzepatide is metabolized by proteolytic cleavage of the peptide backbone, beta-oxidation of the C20 fatty di-acid moiety, and amide hydrolysis | US FDA package insert |
| Excretion | DrugBank | |
| AMES Test (Carcinogenic Effect) | US FDA package insert | |
| hERG Safety Test (Cardiac Effect) | N/A | N/A |
| Liver Toxicity | N/A | N/A |
Since Tirzepatide delays gastric emptying the absorption of other medications and nutrients may be affected and should be monitored by healthcare providers.
Drug Interactions and Side Effects
Table 4. Drug interactions and side effects of Tirzepatide
| Features | Comment(s) | Source |
| Total Number of Drugs Interactions | Over 240 drugs | Drugs.com |
| Major Drug Interactions | bexarotene and gatifloxacin | Drugs.com |
| Alcohol/Food Interaction(s) | moderate interaction with alcohol (ethanol) | Drugs.com |
| Disease Interaction(s) | thyroid carcinoma (major), pancreatitis (moderate), renal dysfunction (moderate), severe GI disease (moderate), retinopathy (moderate) | Drugs.com |
| On-site Binding Side Effects | N/A | N/A |
| Off-site Binding Side Effects | N/A | N/A |
| CYP Interactions | low potential for pharmacokinetic drug-drug interactions related to cytochrome P450 (CYP) | US FDA package insert |
Regulatory Approvals/Commercial
Developed by Eli Lilly and approved by the US FDA in 2022, Mounjaro (Tirzepatide) is prescribed as a subcutaneous pen-injector. Used along with a diet and exercise regime, Tirzepatide helps control blood glucose levels in individuals with type 2 diabetes and also reduce weight in individuals with weight-related medical problems. In a series of clinical trials (SURPASS 1-5) individuals with type 2 diabetes have shown reduction in both HbA1c (1.2 to 2.6%) and body weight (5.4–11.7 kg) (Nauck and D'Alessio 2022).
Although it has a high efficacy in lowering HbA1c, Tirzepatide may have some significant side effects that should be considered when including it in a therapeutic plan.
Links
Table 5. Links to Relevant Resources
| DrugBank | https://go.drugbank.com/drugs/DB15171 |
| Drugs.com | https://www.drugs.com/tirzepatide.html |
| Food and Drugs Administration | https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215866s000lbl.pdf |
| Liver Tox: National Institutes of Health (NIH) | https://www.ncbi.nlm.nih.gov/books/NBK581694/ |
References
Chavda, V.P., Ajabiya, J., Teli, D., Bojarska, J., Apostolopoulos, V. (2022) Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules. 27, 4315. https://doi.org/10.3390/molecules27134315
Conlon, J.M., O'Harte, F.P.M., Flatt, P.R.. (2022) Dual-agonist incretin peptides from fish with potential for obesity-related Type 2 diabetes therapy - A review. Peptides. 147, 170706. https://doi.org/10.1016/j.peptides.2021.170706
Knerr, P.J., Mowery, S.A., Finan, B., Perez-Tilve, D., Tschöp, M.H., DiMarchi, R.D. (2020) Selection and progression of unimolecular agonists at the GIP, GLP-1, and glucagon receptors as drug candidates. Peptides. 125, 170225. https://doi.org/10.1016/j.peptides.2019.170225
Nauck, M.A., D'Alessio, D.A.. (2022) Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc Diabetol. 21, 169. https://doi.org/10.1186%2Fs12933-022-01604-7
Nowak, M., Nowak, W., Grzeszczak, W. (2022) Tirzepatide - a dual GIP/GLP-1 receptor agonist - a new antidiabetic drug with potential metabolic activity in the treatment of type 2 diabetes. Endokrynol Pol. 73(4):745-755. https://doi.org/10.5603/ep.a2022.0029
Sun, B., Willard, F.S., Feng, D., Alsina-Fernandez, J., Chen, Q., Vieth, M., Ho, J.D., Showalter, A.D., Stutsman, C., Ding, L., Suter, T.M., Dunbar, J.D., Carpenter, J.W., Mohammed, F.A., Aihara, E., Brown, R.A., Bueno, A.B., Emmerson, P.J., Moyers, J.S., Kobilka, T.S., Coghlan, M.P., Kobilka, B.K., Sloop, K.W. (2022) Structural determinants of dual incretin receptor agonism by tirzepatide. Proc Natl Acad Sci U S A. 119, e2116506119. https://doi.org/10.1073/pnas.2116506119
August 2023 Dr. Shuchismita Dutta; Reviewed by Dr. Joseph D. Ho
http://dx.doi.org/10.2210/rcsb_pdb/GH/DM/drugs/in/Tirzepatide



