Molecule of the Month: Click Chemistry
A modular approach to chemistry simplifies the construction of complex protein-targeting molecules.
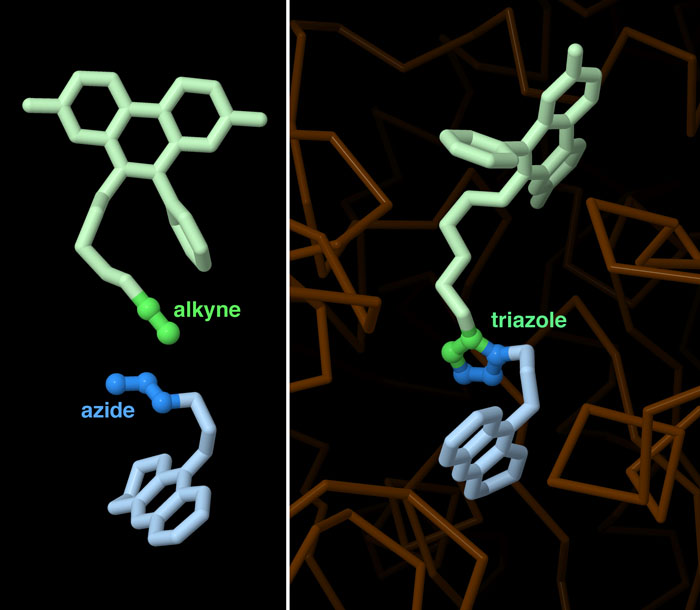
Molecular Legos
Let the Enzyme Choose
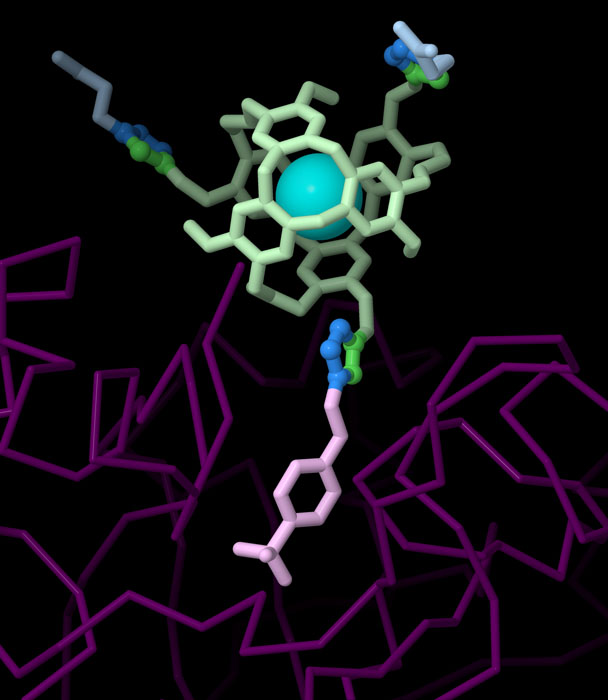
Exotic Constructions
Exploring the Structure
Click Chemistry Enzyme Inhibitor

Click chemistry between azide and alkyne precursors can generate two slightly different triazoles, anti and syn, depending on how they line up next to each other during the reaction. When catalyzed by copper, most of the triazoles have the anti configuration, with the two connected precursors extending in opposite directions from the five-membered ring (PDB ID 1q84). However, in the acetylcholinesterase-catalyzed reaction described above, the precursors mostly align side-by-side to form the syn triazole (PDB ID 1q83). As expected, biochemical testing showed that this syn compound binds more tightly to the enzyme than the anti compound. To explore these two structures in more detail, click on the image for an interactive JSmol.
Topics for Further Discussion
- Many click chemistry inhibitors are included in the PDB archive. You can use the Chemical Sketch Tool in Advanced Search to find them. For example, try searching with the SMILES string CN1C=C(C)N=N1.
- You can see alkyne and azide precursors bound to acetylcholinesterase in PDB ID 5eih.
Related PDB-101 Resources
- Browse Nobel Prizes and PDB structures
- Browse Drug Action
References
- 3cyu: Aaron, J.A., Chambers, J.M., Jude, K.M., Di Costanzo, L., Dmochowski, I.J., Christianson, D.W. (2008) Structure of a 129Xe-cryptophane biosensor complexed with human carbonic anhydrase II. J Am Chem Soc 130: 6942-6943
- Prescher, J.A., Bertozzi, C.R. (2005) Chemistry in living systems. Nat Chem Biol. 1: 13-21
- 1q83, 1q84: Bourne, Y., Kolb, H.C., Radic, Z., Sharpless, K.B., Taylor, P., Marchot, P. (2004) Freeze-frame inhibitor captures acetylcholinesterase in a unique conformation. Proc Natl Acad Sci U S A 101: 1449-1454
- Kolb, H.C., Sharpless, K.B. (2003) The growing impact of click chemistry on drug discovery. Drug Discov Today 8:1128-1137
- Lewis, W.G., Green, L.G., Grynszpan, F., Radic, Z., Carlier, P.R., Taylor, P., Finn, M.G., Sharpless, K.B. (2002) Click chemistry in situ: Acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew Chem Int Ed 41: 1053-1057
December 2022, David Goodsell
http://doi.org/10.2210/rcsb_pdb/mom_2022_12


