Fosfomycin Resistance
Susceptibility Testing
When possible, antibacterial substances, such as fosfomycin, are tested for their effectiveness against various infectious pathogens. These test results allow clinicians to choose the antibiotic likely to result in the most effective treatment of a particular bacterial infection. For instance, one such susceptibility test provides minimum inhibitory concentration (MIC) values that can then be used to identify a pathogenic bacterial strain as susceptible, intermediate, or resistant to a certain antibiotic (see Table 6).
Table 6. Minimum inhibitory concentrations (MIC) that would classify the pathogenic bacterial strain as susceptible, intermediate, or resistant (FDA, 2008). These concentrations may not be the latest, approved by the US FDA.
| MIC (µg/mL) for Susceptible (S) strains | MIC (µg/mL) for Intermediate (I) strains | MIC (µg/mL) Resistant (R) strains |
|---|---|---|
| ≤ 64 | 128 | ≥ 256 |
Fosfomycin Resistance Mechanisms
Fosfomycin resistance occurs when the antibiotic is not able to treat certain bacterial infections because the pathogens causing these infections have developed mechanisms to prevent the drug from functioning. For fosfomycin specifically, there are several mechanisms of bacterial resistance (Silver, 2017). The main ones include:
* Antibiotic modification
* Antibiotic target alteration
* Antibiotic efflux
Antibiotic Modification
When fosfomycin, enters the bacterial cell, it may bind to various metallo-enzymes that add chemical groups to modify it. For example:
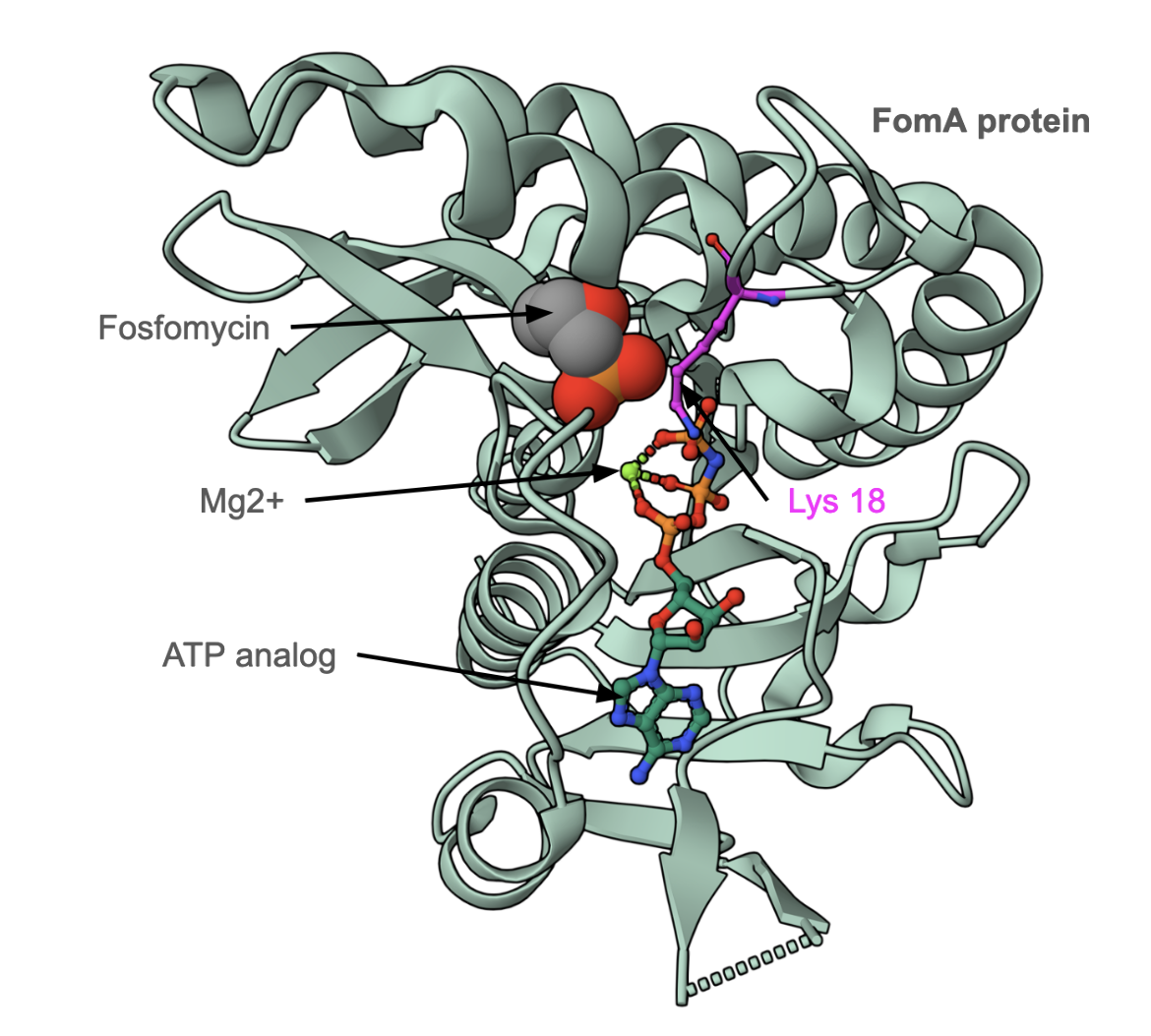
The FomA gene product is a kinase that phosphorylates fosfomycin in the presence of ATP and magnesium (II). Figure 6 shows the structure of fosfomycin resistance kinase FomA from Streptomyces wedmorensis complexed with an ATP analog, Mg2+, and fosfomycin. The Lys18 residue stabilizes the transition state complex during the antibiotic phosphorylation reaction. Addition of the phosphate group inactivates the antibiotic.
Learn about other examples of resistance by adding chemical groups to antibiotics.
|
| Figure 6: Structure of fosfomycin resistance kinase FomA from Streptomyces wedmorensis (PDB ID 3d41, Pakhomova et al., 2008) bound to fosfomycin, Mg2+ and an ATP analog. |
Antibiotic Inactivation
When fosfomycin, enters the bacterial cell, it may bind to it and break specific bonds to inactivate it. For example:
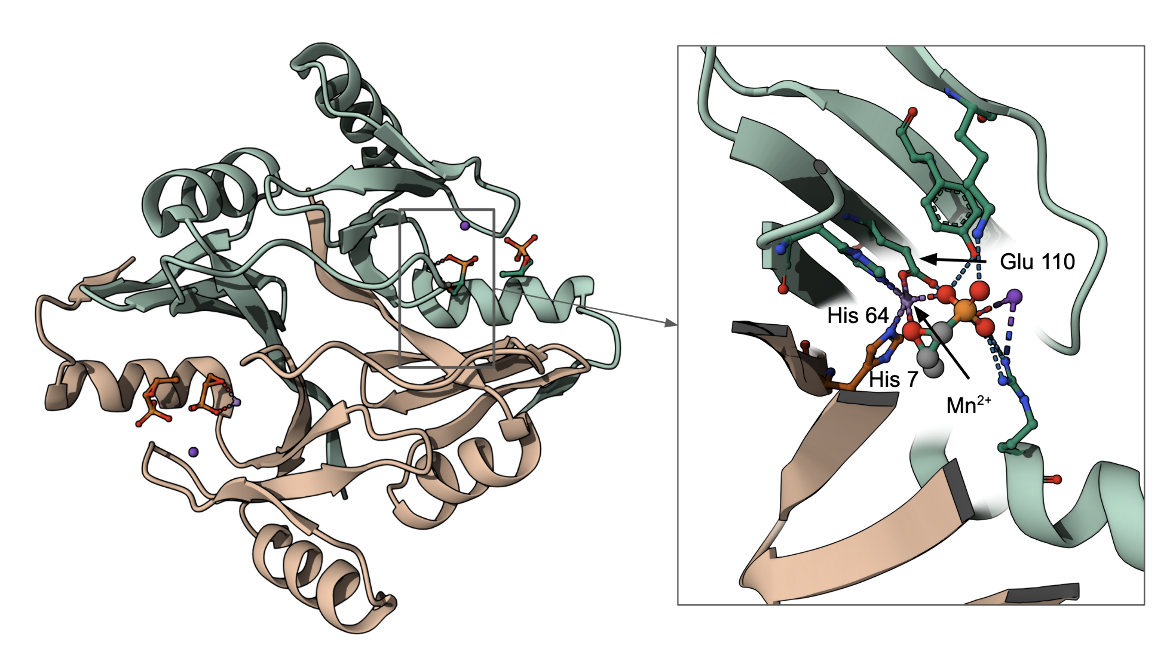
The FosA gene product in Pseudomonas aeruginosa catalyzes the conjugation of glutathione to carbon-1 of fosfomycin and breaks the epoxide ring, rendering it ineffective as an antibacterial drug (see Figure 7). The Glu110 residue functions as the proton donor/acceptor in the enzyme. This along with two His residues (one each from both copies of proteins) in this dimeric assembly coordinate a Mn2+ ion and play key roles in the catalysis.
A similar epoxide ring-breaking reaction is catalyzed by the FosB enzyme in the presence of bacillithiol, and FosX in the presence of water molecules.
Learn more about other examples of antibiotic inactivation by breaking bonds.
Antibiotic Target Alteration
The MurA homolog of Mycobacterium tuberculosis (M. tuberculosis) has a naturally occurring aspartate (Asp) residue in the place of a catalytic cysteine (Cys) residue at position 117, and this allows the bacteria to be intrinsically resistant to fosfomycin; nevertheless, the enzymatic function is not compromised. Replacing that Asp with a Cys reverses this Fosfomycin resistance (De Smet et al., 1999). Other mutations in MurA have also been linked to Fosfomycin resistance (Fu et al., 2016).
Although not a direct target of the antibiotic, fosfomycin binds to and is transported across the bacterial cell membrane by transporter proteins. Any alterations in these transporters can also impact the antibiotic's function.
In E. coli, fosfomycin reaches its intracellular target via two uptake systems: the hexose-6-phosphate transport system (UhpT) and the L-alpha-glycerophosphate transport system (GlpT). GlpT and UhpT are normally involved with the uptake of many phosphorylated sugars. Point mutations in these GlpT and UhpT transporters result in reduced import of the drug into the bacteria and ultimately confer resistance to the antibiotic.
Both the transporters (GlpT and UhpT) are positively regulated by cyclic-AMP (cAMP), which is a derivative of ATP and is used for intracellular signal transduction in many different organisms. Specifically, in bacteria as cAMP levels rise, the concentration of binding protein-cAMP complexes also increases, As a result, there are more target sites in DNA that are occupied, and many of these target sites are located within or near promoters that drive the expression of proteins needed for carbon source uptake (Chen, 2022). Since fosfomycin is a mimic of the natural carbon sources that would be transported through these transporters, greater fosfomycin uptake is associated with higher cAMP levels (Silver, 2017). However, when cAMP levels are lowered by mutations in two genes ptsI (phosphoenolpyruvate-protein phosphotransferase) and cyaA (adenylate cyclase) genes, high frequencies of fosfomycin resistance is observed in vitro (Silver, 2017). In vivo, it was found that all the studied resistant bacterial strains had almost exclusive mutations in the genes coding for the two transporters, glpT and/or uhpT, with no mutations found in the cAMP regulatory loci (Silver, 2017).
Resistance can also occur from mutations in genes involved in cyclic AMP synthesis, which regulates glpT expression. For example, mutations in cya genes, which encode adenylate cyclases, can negatively affect the expression of glpT and mutations in ptsI genes for phosphotransferases can negatively affect the expression of glpT and UhpT.
Learn more about species-specific variations and other examples of target gene alterations.
Antibiotic Efflux
Some resistant bacterial cells confer resistance against fosfomycin by transporting the drug out of the cell through efflux pumps. Some of the examples of resistant bacteria and their efflux pumps are described in Table 7 (CARD, ARO:0000025).
Table 7: Bacterial pumps responsible for efflux of fosfomycin.
| Cause of Resistance | Description |
|---|---|
| MdtG protein | This major facilitator superfamily (MFS) antibiotic efflux pump in Escherichia coli when over-expressed, increases fosfomycin and deoxycholate resistance. The mdtG gene is a member of the marA-soxS-rob regulon, and the MdtG protein is also named YceE. (ARO:3001329) |
| AbaF protein | This MFS antibiotic efflux pump, originally identified in Acinetobcater baumannii resulted in increased resistance to fosfomycin, while disruption of this pump increased fosfomycin susceptibility. (ARO:3004573) |
Learn more about efflux pumps.
Back to the article on Fosfomycin.
References
Chen, T., Zhao, L., Liu, Y., Wang, Y., Jian, Y., Zhao, N., Yang, Z., Wang, X., Liu, Q., Li, M. (2022) Mechanisms of high-level fosfomycin resistance in Staphylococcus aureus epidemic lineage ST5. J Antimicrob Chemother. 77, 2816-2826. https://doi.org/10.1093/jac/dkac236
Fu, Z., Ma, Y., Chen, C., Guo, Y., Hu, F., Liu, Y., Xu, X., Wang, M. (2016) Prevalence of Fosfomycin Resistance and Mutations in murA, glpT, and uhpT in Methicillin-Resistant Staphylococcus aureus Strains Isolated from Blood and Cerebrospinal Fluid Samples. Front Microbiol. 6,1544. https://doi.org/10.3389/fmicb.2015.01544
De Smet, K. A. L., Kempsell, K. E., Gallagher, A., Duncan, K., Young, D. B. (1999) Alteration of a single amino acid residue reverses fosfomycin resistance of recombinant MurA from Mycobacterium tuberculosis. Microbiology (Reading). 145, 3177-3184. https://doi.org/10.1099/00221287-145-11-3177
Jia, B., Raphenya, A. R., Alcock, B., Waglechner, N., Guo, P., Tsang, K. K., Lago, B. A., Dave, B. M., Pereira, S., Sharma, A. N., Doshi, S., Courtot, M., Lo, R., Williams, L. E., Frye, J. G., Elsayegh, T., Sardar, D. Westman, E. L., Pawlowski, A. C., Johnson, T. A., Brinkman, F. S., Wright, G. D., and McArthur, A. G. (2017) CARD 2017: expansion and model-centric curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Research 45, D566-573. https://doi.org/10.1093/nar/gkw1004
Pakhomova, S., Bartlett, S. G., Augustus, A., Kuzuyama, T., Newcomer, M. E. (2008) Crystal structure of fosfomycin resistance kinase FomA from Streptomyces wedmorensis. J Biol Chem. 283, 28518-26. https://doi.org/10.1074/jbc.M803709200
Rife, C. L., Pharris, R. E., Newcomer, M. E., Armstrong, R. N. (2002) Crystal structure of a genomically encoded fosfomycin resistance protein (FosA) at 1.19 A resolution by MAD phasing off the L-III edge of Tl(+). J Am Chem Soc. 124, 11001-3. https://doi.org/10.1021/ja026879v
Silver, L. L. (2017) Fosfomycin: Mechanism and Resistance. Cold Spring Harb Perspect Med. 7:a025262. https://doi.org/10.1101/cshperspect.a025262