Efflux Pumps
Introduction
Bacteria can prevent an antibiotic from reaching its target by actively transporting the antibiotic out of the cell using efflux pumps or decreasing the uptake of the antibiotic so that the drug cannot penetrate the bacterial membrane in the first place (Munita et al., 2016). Efflux pumps are membrane transporters, present in all living beings. They use energy, either in the form of ATP or the proton gradient to pump molecules against their concentration gradient. All efflux pumps span at least one membrane and expel molecules from the cytoplasm. In gram-negative bacteria, some efflux pumps are comprised of multiple transporter proteins that interact together and span across both inner and outer membranes. All efflux pumps are molecular machines that require a substantial investment of energy and material to build and operate. While efflux pumps transport many different molecules, they are clinically important too - in human cells, they can lead to resistance to anti-cancer and other drugs, and in bacteria, they can lead to antibiotic resistance (Hernando-Amado et al., 2016). The latter scenarios are described here.
Normal functions of efflux pumps
Some efflux pumps are expressed constitutively and play key roles in biology, while others may be triggered by environmental factors (Du et al., 2015). For example, the Bacillus subtilis multidrug transporter, Blt, expels cationic molecules like polyamine spermidine. Similarly, some transporters in Escherichia coli pump out fatty acids or gastrointestinal bile salts. In contrast, efflux transporter genes are induced in Mycobacterium tuberculosis once it has entered the macrophages to pump out rifampin (Du et al., 2015).
Types of efflux pumps
Efflux pumps can be divided into five families (Table 1) based on size, composition, and energy source (Sun et al., 2014). Some of these pumps are multidrug resistant, while others extrude specific classes of antibiotics. Most efflux pumps have just one independent unit, but all RND pumps and some MFS and ABC systems are tripartite, making them structurally intricate and functionally efficient. Though all five of these families are widespread in bacteria, they are not all clinically relevant in the same species (Du et al., 2015).
Table 1: Classification of the five different families of efflux pumps. Data was collected and synthesized from the CARD database (Jia et al., 2017), and other sources (Piddock 2006a, and Du et al., 2015).
| Family | Distribution | Energy Source | Structure | Clinical relevance | Examples |
|---|---|---|---|---|---|
| ATP binding cassette (ABC) | gram positive or negative; Eukaryotic | ATP | tripartite or independent unit | most widely multi-drug resistance | RanARanB; VRA-F; see also ARO:0010001 |
| major facilitator superfamily (MFS) | gram positive or negative | proton (H+) gradient | tripartite or independent unit | clinically relevant in gram-positive bacteria only | tet family; see also ARO:0010002 |
| small multidrug resistance (SMR) | gram positive or negative | proton (H+) gradient | independent unit | - | KpnEF, see also ARO:0010003 |
| multidrug and toxic compound extrusion (MATE) | gram positive or negative | sodium (Na+) or proton (H+) gradient | independent unit | - | Mdtk; see also ARO:3000112 |
| resistance nodulation cell division (RND) | gram negative only | proton (H+) gradient | tripartite only | often found encoded on chromosomal DNA | AcrAB-TolC; MexAB-OprM; see also ARO:0010004 |
In summary, there are many flavors of bacterial drug efflux pumps. Some, like the ATP-binding cassette (ABC) family, directly utilize ATP as the energy source to drive transport. Others, like the major facilitator superfamily (MFS), multidrug and toxin extrusion (MATE), small multidrug resistance (SMR), and resistance-nodulation-cell division (RND) superfamily, are powered by electrochemical gradients across the membrane. These classes of efflux pumps are described with examples herein.
ATP Binding Cassette (ABC) superfamily
This group of transporters is an ancient superfamily, found in both eukaryotes and prokaryotes. They transport a range of substrates from metal ions to proteins across the membrane and are powered by ATP hydrolysis (Akhtar and Turner, 2022). ABC transporters may either function as an importer or an exporter. Importers in this superfamily facilitate the acquisition of various essential compounds such as metal ions and proteins from the extracellular matrix into the cell's cytoplasm. Exporters translocate various proteins, toxins, and xenobiotics out of the cell (Akhtar and Turner, 2022).
The ABC transporters typically consist of two core units - the nucleotide-binding domain (NBD), and the membrane domain (MD). The NBDs are intracellular and form a dimeric structure. They each bind to and hydrolyze ATP. The MD part of the protein is embedded in the membrane providing a path for the substrate across the membrane (Du et al., 2015). The organization of ABC transporters may vary in different ways - i.e., the NBD-MD subunit(s) may be encoded separately or as a single polypeptide, but the transport mechanism is similar (Du et al., 2015).
Examples of ABC transporters include:
* EfrAB in Enterococcus faecium leads to rifampicin resistance
Here we describe the structure of Sav1866 from Staphylococcus aureus as an example of the multidrug-resistant ABC transporter.
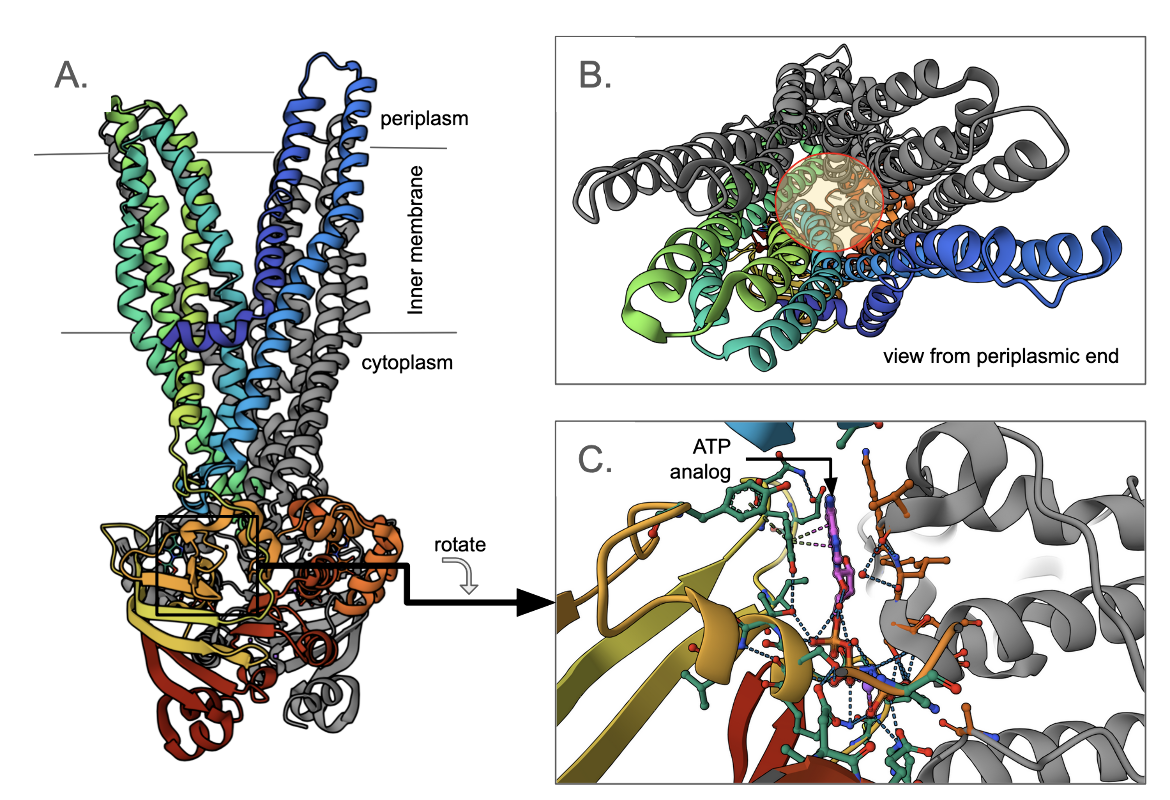
Multidrug transporter Sav1866
The Staphylococcus aureus Sav1866 is homologous to the human ABC transporter Mdr1, causing multidrug resistance in cancer cells. Each copy of the transporter, has a transmembrane N-terminal domain and a cytoplasmic, C-terminal, ATP binding domain. Two copies of the transporter interact with each other, to form a dimer (Figure ABC1A). Here one monomer is colored grey, while the other is represented in the rainbow color scheme (where the N-terminus is blue, the C-terminus is red, and amino acids between the two ends are colored to match the visible spectrum). Note that the N-terminus of one chain is split into two helical bundles of two and four helices (Figure ABC1B). The other monomer forms similar groupings of the helices resulting in two groups of six helices, and a path for the substrates in the middle (shown with a yellow shaded circle). The cytoplasmic C-terminal domain has an ATP analog bound to it. A closeup of this ATP analog shows that it is bound at the dimer interface in the nucleotide-binding domain (Figure ABC1C).
Only the ATP-bound state of both copies of the ABC transporter induces the outward-facing conformation of the transmembrane domains (Dawson and Locher, 2007). Hydrolysis of the ATPs and release of both ADP/Pi leads to NBD dissociation, and resets the transporters to the inward-facing conformation (Du et al., 2015). The alternating access model suggests that the substrate-binding pocket (in the transmembrane part) is exposed to the interior and exterior of the cell as it alternates between inward-facing and outward-facing conformations. Whether the ATPs bound to the homodimeric ABC transporters hydrolyze simultaneously, sequentially, or in an alternating fashion has not yet been resolved in detail.
In gram-negative bacteria, ABC efflux transporters can also form tripartite efflux pumps, together with a membrane fusion protein and an outer membrane protein. For example, in E. coli MacAB, MacB is the inner membrane transporter, MacA is the membrane fusion protein, and TolC traverses the outer membrane to form a tripartite complex (Hernando-Amado et al., 2016).
Major Facilitator Superfamily (MFS)
These transporters control the uptake of nutrients and excretion of waste in bacterial, archaeal, and eukaryotic cells. They transport simple sugars, inositols, amino acids, nucleosides, and a broad spectrum of chemically unrelated drugs (e.g., chloramphenicol, erythromycin, certain aminoglycosides, and fluoroquinolones) (Edgar and Bibi, 1997).
Typical major facilitator superfamily proteins are made up of twelve transmembrane helices. They facilitate the movement of substances by flexing back and forth between a conformation that is open to the inside of the cell (cytoplasm), and one which is open to the periplasm (Nagarathinam et al., 2018). This movement is defined as the “rocker switch mechanism” and is energized by the electrochemical proton gradient.
Examples of MFS transporters include
* AbaF in Acinetobacter baumannii leads to fosfomycin resistance
* EfpA in Mycobacterium tuberculosis leads to rifampicin resistance
* KpnGH-TolC in Klebsiella pneumoniae leads to azithromycin resistance, gentamicin resistance, and streptomycin resistance
* LmrS in Staphylococcus aureus leads to trimethoprim resistance, linezolid resistance, and streptomycin resistance
* MdtG in E. coli leads to fosfomycin resistance
* Mef(B) in E. coli and Streptococcus agalactiae lead to azithromycin resistance
* NorB in Staphylococcus aureus leads to fluoroquinolone resistance and other structurally unrelated antibiotics such as tetracycline.
* QepA2 in E. coli leads to fluoroquinolone resistance and several classes of other antibiotics including tetracyclines, oxazolidinones, macrolides, and lincosamides
* Tet(A), Tet(B), Tet(K) in many gram-negative bacteria leads to tetracycline resistance
Here we describe the structure and function of MdfA from Escherichia coli as an example of the major facilitator superfamily pump.
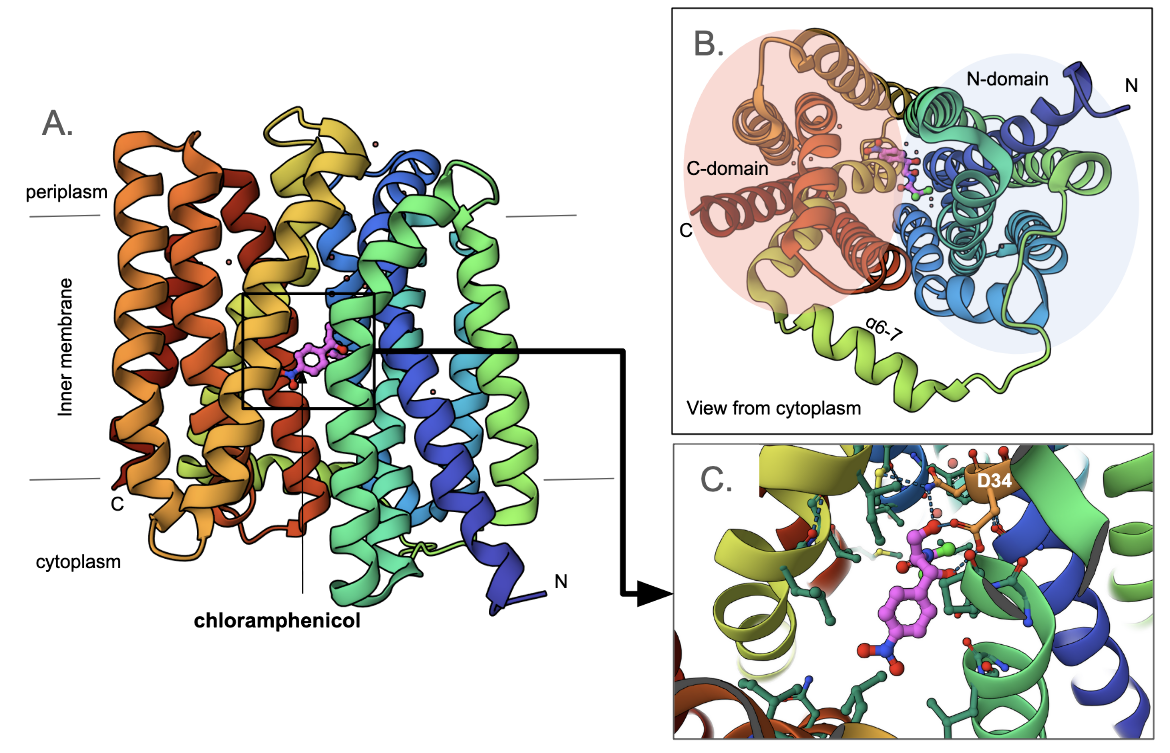
Multidrug transporter MdfA
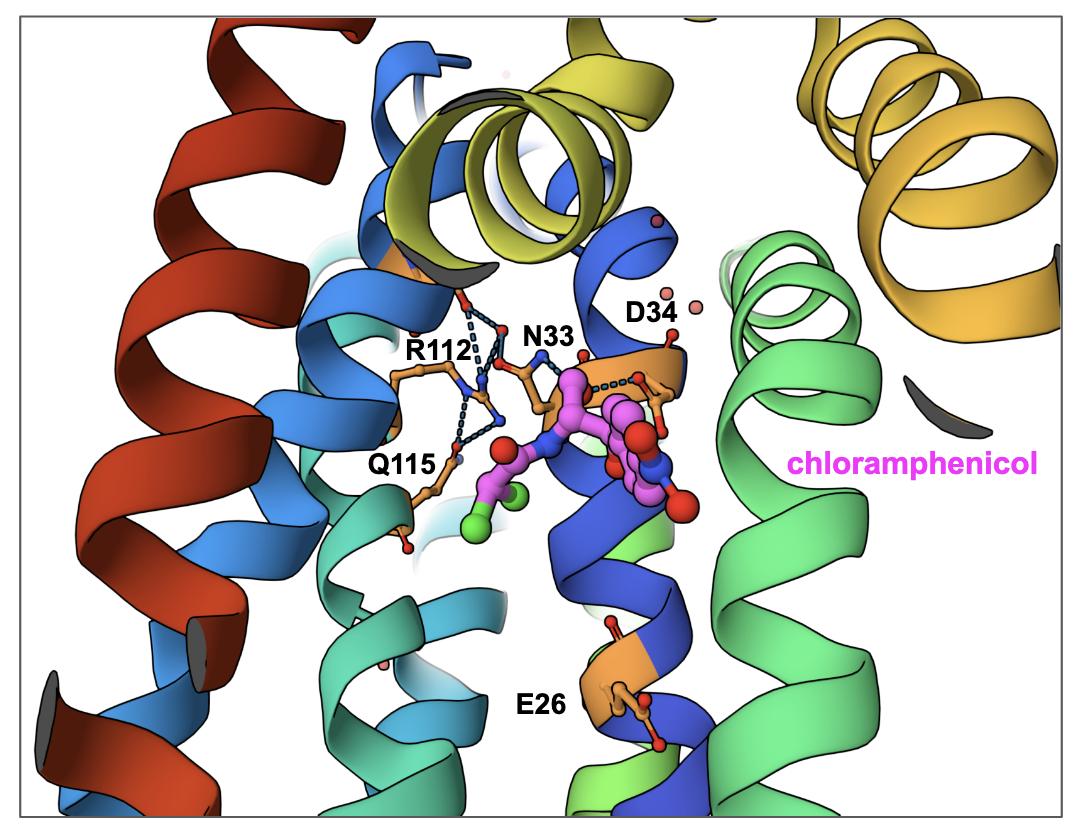
The mdfA gene found in Escherichia coli codes for a transporter with a 12 transmembrane (TM)-helix core (Figure MFS1A). Viewing it from the cytoplasmic face it is clear that it consists of two pseudo-symmetrical, six-helix domains (N-domain and C-domain, Figure MFS1B). The central cavity between these two domains forms the path for substrate transport. Here a chloramphenicol molecule is shown, bound to the inward-facing pump. Note that the antibiotic is bound closer to the N-domain (compared to the C-domain). A closeup of the antibiotic binding shows that it forms hydrogen bonds with two adjacent amino acids Asn33 and Asp34. Other amino acids surrounding the cavity are hydrophobic in nature. The Asp34 is conserved in this class of transporters. Mutating it to Ala or Asn abolished antibiotic resistance (Heng et al., 2015).
In addition to Asp34, another proton-titratable residue inside the central cavity - Glu26 is shown. This plays a key role in binding the proton when the transporter is in its outward-facing conformation. Mutating the Glu to Ala or Gln abolished antibiotic resistance (Heng et al., 2015). Finally, a conserved motif R112-x-x-Q115 sits at the back wall of the antibiotic binding cavity. The positively charged residue Arg112 forms hydrogen bonds with Gln115 and water-mediated hydrogen bonds with Asn33. These interactions are thought to play a key role in the transporter's function (Figure MFS2). Substituting the Arg with His or Lys retains the resistance phenotype while replacing it with Met, Gln, or Glu abolishes it (Heng et al., 2015).
Overexpression of MdfA from plasmids has been observed in multidrug-resistant E. coli strains isolated from clinical patients (Heng et al., 2015).
|
| Figure MFS2. A closeup of the back wall of the central cavity of MdfA with bound chloramphenicol (PDB ID 4zow, Heng et al., 2015). Key residues that play a role in its function are shown. |
Small Multidrug Resistance (SMR) superfamily
These transporters use proton motive force to extrude toxic compounds including quaternary ammonium compounds, toxic lipophilic compounds, DNA intercalating dyes, toxic metabolites - e.g., nicotine intermediates, and polyamine compounds like spermidine (Hernando-Amado et al., 2016). Genes coding for SMR proteins (e.g., emrE, sugE, smr, qac, ebr) are often found in mobile elements. This provides a selective advantage to microbes allowing them to survive in and develop resistance to ubiquitous environmental pollutants with low-grade toxicity (Kermani et al., 2020). The adaptive effects of the SMR proteins lead to co-selection of other genes that confer resistance to the antimicrobial arsenal, including sulfonamides, β-lactams, and aminoglycosides, increasing the frequency of these genes in environmental reservoirs (Kermani et al., 2020).
Members of the SMR family consist of short polypeptides (100–150 amino acids) and span the cytoplasmic membrane as four transmembrane α-helices. Two of these proteins form a dimer to make the functional transporter.
Examples of SMR transporters include:
* KpnEF in Klebsiella pneumoniae leads to tetracycline resistance, streptomycin resistance, and rifampicin resistance
*YkkCD also leads to streptomycin resistance
Here we discuss EmrE from Escherichia coli as an example of the small multidrug resistance superfamily.
EmrE multidrug transporter
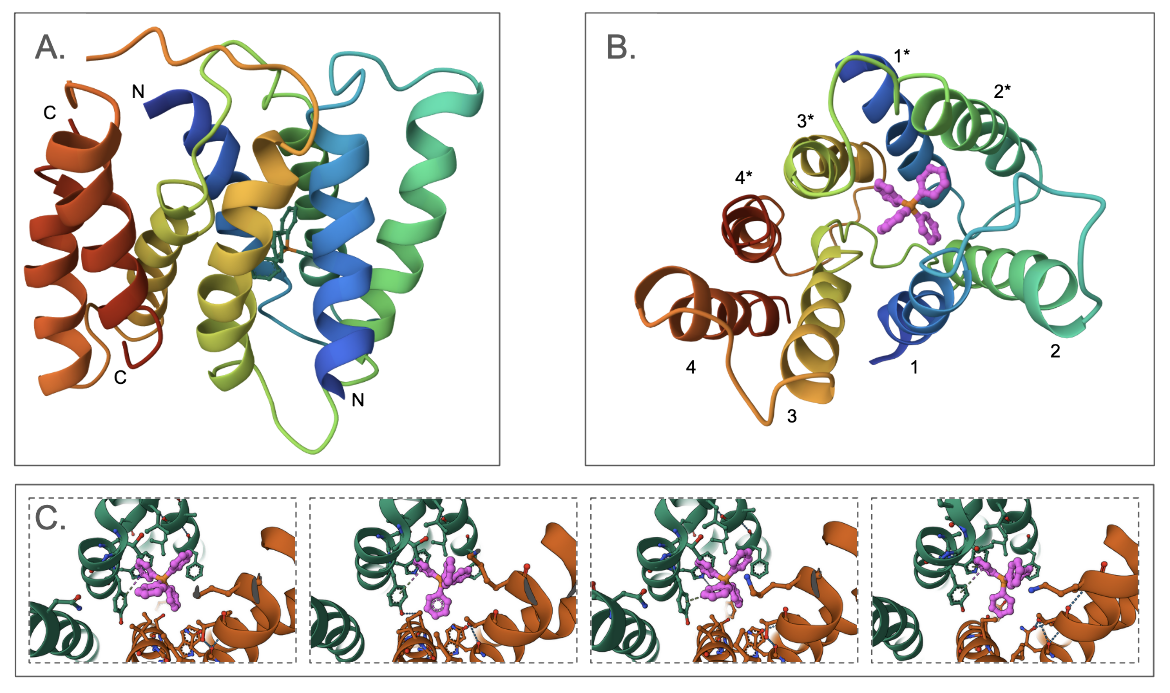
The EmrE multidrug transporter forms a homodimer - where each monomer has four transmembrane helices. Interestingly, the two monomers are antiparallel - i.e., the N- and C-termini of each monomer are oriented towards opposite ends (Figure SMR1A). The first three transmembrane helices from each monomer surround the substrate binding pocket, where the tetraphenylphosphonium (TPP) is bound. The last helix of each monomer interacts together to form the dimer (Figure SMR1B). The structure shown here (PDB ID 3b5d) is a low-resolution structure with no coordinates for the side chains. To examine the details of the substrate binding, we examine a different structure (determined using NMR, PDB ID 8uoz) and see that there is conformational flexibility in the ligand's conformation and that it is capable of forming a series of different pi-stacking interactions with Tyr60 in the substrate binding site (Figure SMR1C).
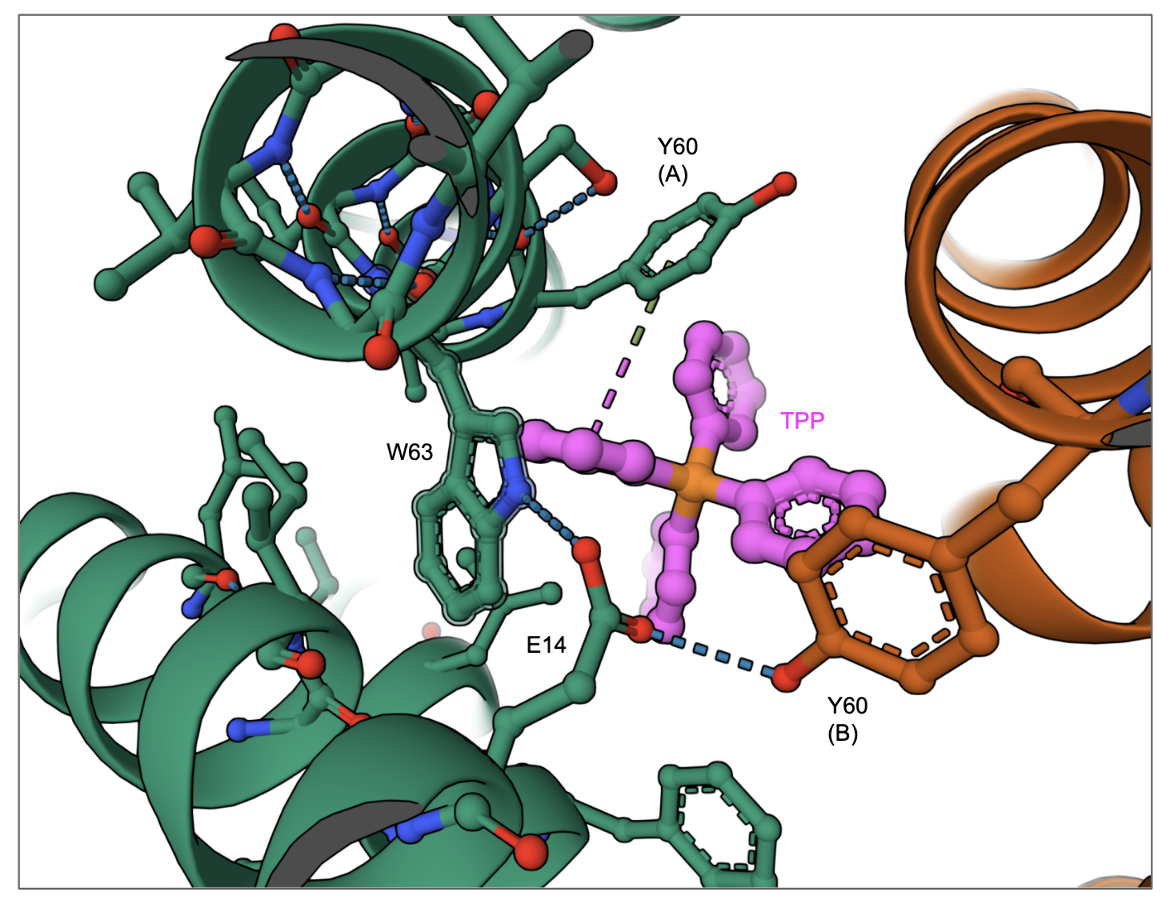
One amino acid Glu14 in EmrE is critical for its function. The Glu14 in one monomer within the membrane forms hydrogen bonds with Trp63 in the same monomer and Tyr60 of the other monomer (Figure SMR2). The protonation and deprotonation of this Glu is thought to facilitate the opening and closing of the transporter allowing the substrate to enter the central chamber for transport (Li et al. 2024).
In M. tuberculosis, the causative agent of tuberculosis, overexpression of an SMR protein, called Mmr, reduces the bacterial susceptibility to ethidium bromide and quaternary ammonium compounds, indicating that these molecules are transported by the Mmr pumps (Rodrigues et al., 2013). However, isoniazid susceptibility was not affected by the absence or overexpression of mmr and needs to be further studied.
|
| Figure SMR2. A closeup of the interactions of TPP in the substrate binding cavity of the EmrE transporter. (PDB ID 8uoz, model 4, Li et al., 2024) |
Multidrug And Toxic compound Extrusion (MATE) superfamily
Members of this group of transporters are widely distributed in bacteria, archaea, and eukarya (Raturi et al., 2021). They confer multidrug resistance in pathogenic microorganisms and affect pharmacokinetics in mammals so they are an important class of transporters.
Typically the multidrug and toxic compound extrusion (MATE) consists of 12 transmembrane helices with an N-lobe and a C-lobe with a pseudo twofold symmetry about an axis perpendicular to the membrane plane (Gaurav et al., 2023). Since these helices are arranged differently from those in major facilitator superfamily (MFS) transporters they were classified into a separate superfamily (Raturi et al., 2021). Transporters in this superfamily are divided into three subfamilies based on specific structural features of their structure - the DinF and NorM subfamilies in prokaryotes, and the eukaryotic MATE subfamilies. While DinF members are antiporters that use the entrance of cations (usually sodium) for the extrusion of the drug, the NorM transporters use protons for the antiport (Hernando-Amado et al., 2016).
Examples of MATE transporters include:
* MepA in Staphylococcus aureus leads to tigecycline resistance.
Here we describe the structure and function of NorM from Vibrio cholerae and DinF-BH from Halalkalibacterium halodurans as examples of the multidrug and toxic compound extrusion superfamily pump.
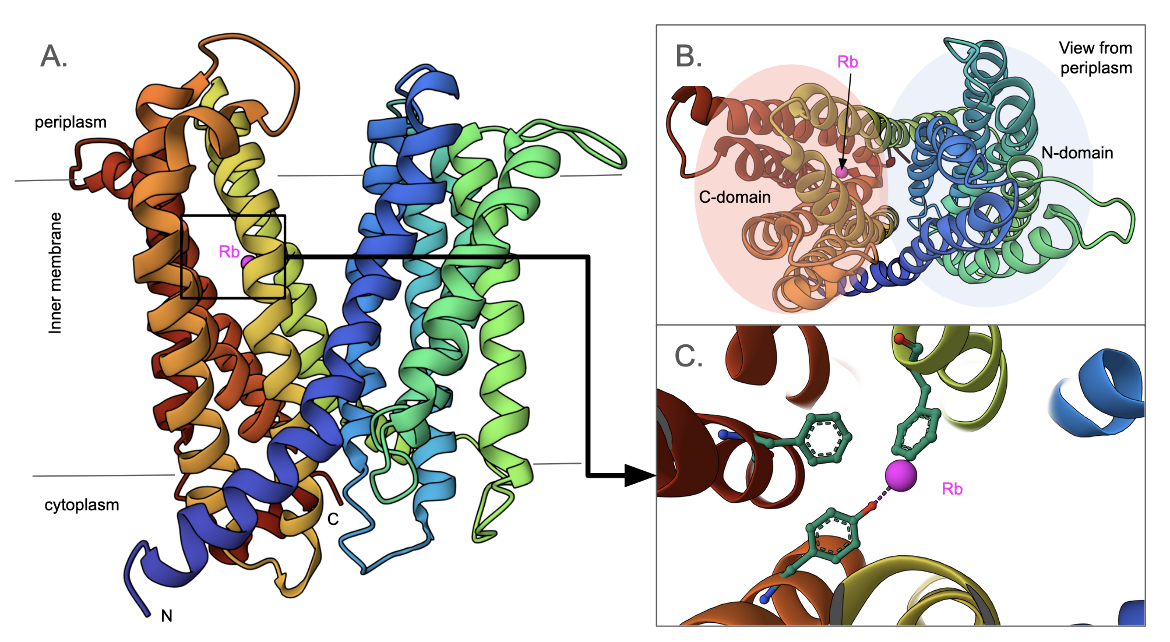
NorM transporter
The structure of the NorM transporter from Vibrio cholerae reveals a 12-transmembrane helical structure with an outward-facing conformation for the transporter (Figure MATE1A, He et al., 2010). When viewed from the periplasmic face, the helices are grouped into N- and C-domains (Figure MATE1B), with a V-shaped channel in between. A cation (Rb+) is bound in between the helices of the C-domain (Figure MATE1C). Three conserved amino acids, critical for the transporter's function map to Asp36, Glu255, and Asp371. These amino acids do not interact with the cation in the structure shown here, however, they likely play key roles in other conformations facilitating transport.
DinF-BH transporter
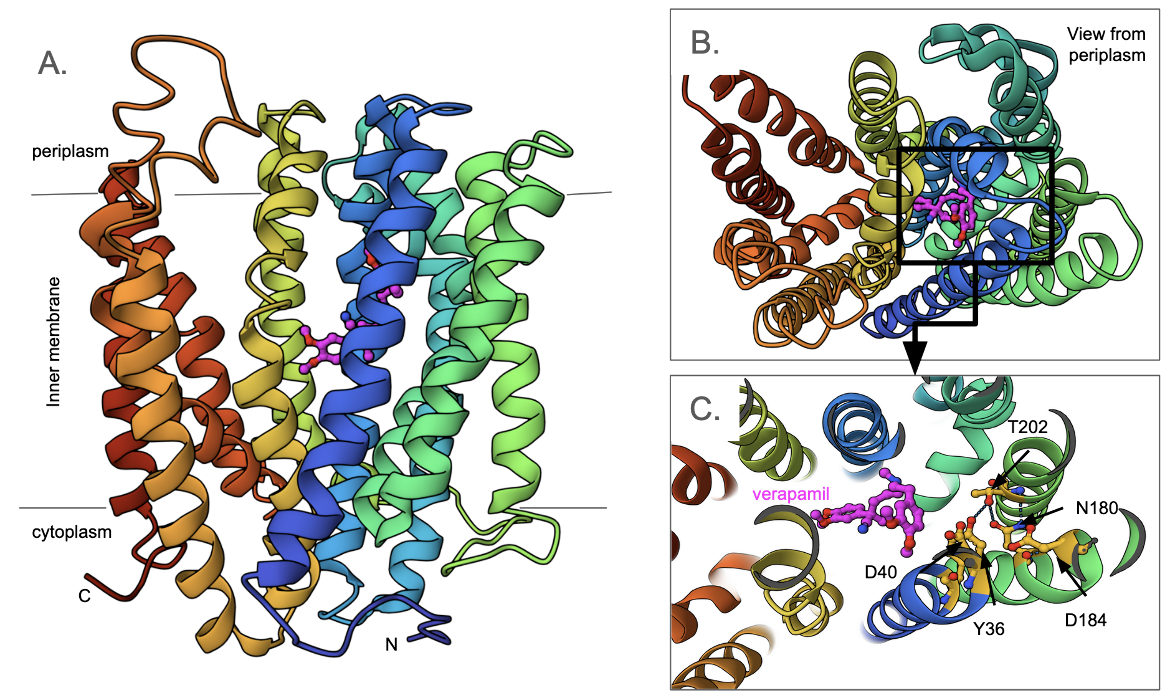
The structure of the DinF-BH transporter from Halalkalibacterium halodurans reveals a 12 transmembrane helical structure organized into N- and C-domains (Figure MATE2A and B). It functions as a proton-coupled multidrug transporter to pump out toxins and drugs. A series of conserved polar and charged amino acids (Asp40, Ans180, Asp184, Thr202, and Tyr36) play a key role in the transporter function (Figure 2C). As is typical for other MATE transporters, both N and C termini face the cytoplasm (Lu et al., 2013). The substrate binding pocket is located in the N-domain while a wide crevice lies within the C-domain. In the structure shown here (PDB ID 5c6o, Radchenko et al., 2015) verapamil, an inhibitor of the transporter is bound to the substrate binding pocket.
Resistance Nodulation cell Division (RND) superfamily
This class of multidrug resistance pumps is found just in gram-negative bacteria (Sun et al., 2014). One of the best-studied pumps of this class, AcrAB-TolC, conveys resistance to tigecycline, chloramphenicol, rifampin, tetracycline, ampicillin, fluoroquinolones, and several other antibiotics (Jia et al., 2017: CARD database).
This type of pump is tripartite in its structure and includes (a) an inner membrane transport protein (e.g., AcrB); (b) a periplasmic fusion protein (e.g., AcrA); and (c) an outer membrane channel protein (e.g., TolC) (Wang et al., 2017) (Fig. 4). Due to this tripartite structure (Figure RND1), the pump can move antibiotics from the cytosol straight through to the extracellular environment without a periplasmic intermediate, so it is highly efficient at extruding antibiotics.
Examples of RND efflux pumps include:
* AbuO in Acinetobacter baumannii leads to streptomycin resistance and tigecycline resistance
* AcrAB-TolC leads to tigecycline resistance and rifampicin resistance
* AcrD in Escherichia coli leads to gentamicin resistance
* AdeABC in Acinetobacter species (notably A. baumannii) leads to tigecycline resistance
* AdeIJK in Acinetobacter baumannii leads to trimethoprim resistance and rifampicin resistance
* AmrAB-OprM in Burkholderia vietnamiensis leads to azithromycin resistance
* LpeAB in L. pneumophila leads to azithromycin resistance
* MexAB-OprM in Pseudomonas aeruginosa leads to tetracycline resistance, azithromycin resistance, trimethoprim resistance, and aztreonam resistance
* MexCD-OprJ with type A NfxB phenotype leads to gentamicin resistance
* MexXY-OprA in Pseudomonas aeruginosa leads to gentamicin resistance
* MexXY-OprM in Pseudomonas aeruginosa leads to gentamicin resistance
* OqxAB in clinical isolates of Enterobacteriaceae leads to trimethoprim resistance and tigecycline resistance
* YajC leads to linezolid resistance
Here we examine the structure and functions of the MexB-MexA-OprM system of P. aeruginosa as an example of a resistance nodulation cell division pump.
MexAB-OprM Drug Efflux Pump
Pseudomonas aeruginosa is a gram-negative and rod-shaped bacteria that mostly infects immunocompromised patients, in hospitals. While various resistance mechanisms include target alteration, target replacement, target protection, antibiotic inactivation, and reduced permeability to antibiotics, the presence of an efflux pump (MexAB-Oprm) allows it to resist structurally and functionally dissimilar antibiotics.
The pump consists of three subunits, MexA, MexB, and OprM (Figure RND1A). It forms a vertically elongated rod with a length of 320 Å and a width of 110 Å. In this structure (PDB ID 6iok, Tsutsumi et al., 2019) we see that
* MexB is the inner membrane component and responsible for selecting substrates and using energy to transport them out of the cell. It selects and transports a broad range of substrates including antibiotics, dyes, detergents, and organic solvents. It forms an asymmetric trimer and uses the proton electrochemical gradient to transport them across the membranes
* OprM is the outer-membrane-anchored lipoprotein and plays a role in the final stage of antibiotic ejection out of the cell. It forms a trimer with threefold symmetry.
* MexA is the membrane fusion protein and binds to MexB and OprM. It spans the periplasmic space and provides a channel for the substrate to pass through. It forms a hexamer with C6 symmetry.
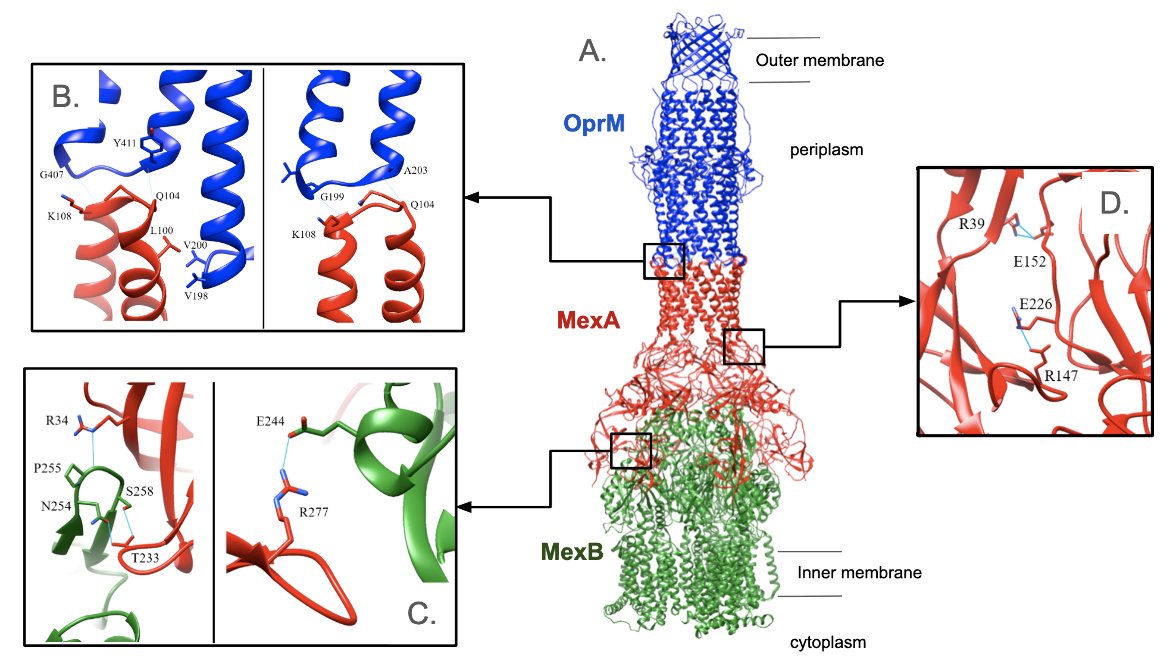
Key interactions between the α-hairpin domain of MexA and OprM occur near the outer membrane of the cell (Figure RND1B). Several hydrogen bonds connect the two proteins: K108 in MexA binds to Gly199 or Gly407 in OprM; Gln104 in MexA binds to Ala203 or Tyr411 in OprM. In addition, Leu100 in MexA makes hydrophobic interactions with Val198 and Val200 in OprM. Since MexA is a hexamer and OprM is a trimer, each interaction occurs three times in the pump due to its symmetry. When Tsutsumi et al. (2019) separately mutated Leu100, Gln104, and Lys108 in MexA, complex formation was hindered, leading to decreased drug resistance. Therefore, it was concluded that these amino acids are critical for the binding of MexA to OprM. However, it was important to confirm that Leu100 is crucial because the 99th amino acid is also leucine. Leu99 was mutated to aspartate but this had no effect on drug resistance which means that it is not essential for complex formation.
Both the β-barrel and membrane-proximal domains of MexA interact with MexB (Figure RND1C). The contact surface area is about 1820 Å2. A loop of MexB between Leu252 and Val260 sticks into a hollow section formed by the β-barrel domain of MexA. Within this loop, Pro255, Asn254, and Ser258 of MexB are at a suitable distance to form hydrogen bonds with R34 and Thr233 of MexA. In a separate location, Arg277 in the membrane-proximal domain of MexA forms a hydrogen bond with Glu244 in MexB. To verify that these amino acids are crucial, R34, Thr233, and Arg277 were separately mutated and the ability to form a complex was lost (Tsutsumi et al., 2019).
The MexA hexamer has C6 symmetry, except for the membrane-proximal domains. Residues in the β-barrel domain form van der Waals interactions over a contact surface area of 1254 Å2. Glu152 or Glu226 interact with Arg39 or Arg147 of the adjacent monomer (Figure RND1D). Point mutations were made to confirm that these amino acids are critical. The positively charged arginine residues were changed to negatively charged aspartic acids. As a result, they failed to interact with negatively charged glutamic acid residues. This prevented complex formation and decreased drug resistance (Tsutsumi et al., 2019).
The MexAB-OprM efflux pump can transport beta-lactams, fluoroquinolones, and sulfonamides, in addition to a highly diverse set of metabolites. The MexXY-OprM pump preferentially transports aminoglycosides and tetracyclines (Zwama et al., 2021). Mutations in the efflux pump regulatory systems leading to pump overexpression are among the most common efflux-related mechanisms for drug resistance described. Meanwhile, amino acid substitutions in known structural binding sites in the IMP subunits of RND efflux pumps appear to have the greatest impact on substrate specificity (Dulanto, Chiang, and Dekker, 2024).
Regulation of Efflux pumps
In addition to learning about the architecture and function of efflux pumps, it is important to understand their regulation, which is complex and highly interconnected. These efflux pump networks complicate the interpretation of resistant phenotypes. There are species-specific variations in these regulations. Even within a species, the physiological status of the bacterial cells can lead to differences in these regulations (Du et al., 2018). Alteration in the regulatory system (e.g., mutations) may lead to overexpression of the same or alternate efflux pumps. For example, the deletion of acrB results in a compensatory overproduction of AcrD and AcrF (Blair et al., 2015).
Two broad classes of resistance regulation mechanisms are discussed here.
Regulation by two-component systems
Bacteria sense various environmental stimuli including the presence of antibiotics or stress associated with drug action and respond adaptively to confer drug resistance. This type of regulation is mediated by combinations of a sensor histidine kinase and response regulator, and is found in many organisms (Du et al., 2018). For example, the histidine kinase VanS senses the antibiotic vancomycin and responds by phosphorylating the VanR or response protein. Learn more about this regulation in the context of the vanA operon. The two-component system seen in B. subtilis presents an example of indirect action - the bacitracin efflux ABC transporter BceAB acts as the sensor by communicating its transport to the histidine kinase BceS through protein-protein interaction (Fritz et al., 2015).
Regulation by transcription and post-transcription factors
This regulation is mediated through mutations in transcriptional repressors or promoters of the genes encoding the RND and ABC transporters. For example, the tetracycline repressor protein (TetR) family of transcriptional repressors (e.g., emrR, acrR, and mtrR), or overexpression of global transcriptional regulators, including AraC–XylS transcription factors (for example, MarA, SoxS, and RamA), results in overexpression of efflux pumps and increased transport activity (reviewed in Du et al., 2018).
The transcription of the MexAB-OprM efflux pump is primarily regulated by MexR, a repressor protein that binds to the promoter of the mexAB-oprM operon (Anandapadamanaban et al., 2016). It belongs to the MarR family of regulators. They can sense and respond to extracellular and intracellular changes to regulate transcription. Several antibiotics induce an oxidative damage pathway that leads to cellular death. It has been shown in prior experiments that MexR senses this oxidative stress when bound to a promoter. Under oxidative conditions, the cystines form a disulphide bond. This causes the dissociation of MexR from the promoter which activates the mexAB-oprM operon. This mechanism details how P. aeruginosa responds to the presence of antibiotics.
Clinical implications of resistance by efflux pumps
In clinical samples with resistant bacteria, current efforts are focused on identifying the resistance genes present in them. While this information can inform the choice of antibiotic used in the treatment plan, there are no easy and reliable methods for determining the types and expression of efflux pumps in the bacteria in these samples. Therefore, it can be challenging to make informed choices about antibiotics for the treatment plans (Davin-Regli et al., 2021). Both clinical and laboratory data indicate that efflux pumps also play key roles in virulence and the adaptive responses that facilitate antimicrobial resistance. So by understanding the structure, function, and regulation of efflux pumps we can uncover opportunities for altering/inhibiting their activities to overcome resistance and restore antibiotic function.
References
Ad hoc Interagency Coordination Group on Antimicrobial Resistance. (2019). No time to wait: securing the future from drug-resistant infections. IACG discussion paper. Retrieved from https://www.who.int/publications/i/item/no-time-to-wait-securing-the-future-from-drug-resistant-infections
Akhtar, A. A., Turner, D. P. (2022) The role of bacterial ATP-binding cassette (ABC) transporters in pathogenesis and virulence: Therapeutic and vaccine potential. Microb Pathog. 171:105734. https://doi.org/10.1016/j.micpath.2022.105734
Anandapadamanaban, M., Pilstål, R., Andresen, C., Trewhella, J., Moche, M., Wallner, B., Sunnerhagen, M. (2016). Mutation-Induced Population Shift in the MexR Conformational Ensemble Disengages DNA Binding: A Novel Mechanism for MarR Family Derepression. Structure (London, England : 1993), 24(8), 1311–1321. https://doi.org/10.1016/j.str.2016.06.008
Blair, J. M., Webber, M. A., Baylay, A. J., Ogbolu, D. O., Piddock, L. J. (2015). Molecular mechanisms of antibiotic resistance. Nature reviews. Microbiology, 13(1), 42–51. https://doi.org/10.1038/nrmicro3380
Centers for Disease Control and Prevention. (2013). Antibiotic resistance threats in the United States, 2013. Retrieved from https://www.cdc.gov/antimicrobial-resistance/media/pdfs/ar-threats-2013-508.pdf
Chen, Y. J., Pornillos, O., Lieu, S., Ma, C., Chen, A. P., Chang, G. (2007) X-ray structure of EmrE supports dual topology model. Proc Natl Acad Sci U S A. 27;104(48):18999-9004. https://doi.org/10.1073/pnas.0709387104
Davin-Regli, A., Pages, J. -M., Ferrand, A. (2021) Clinical Status of Efflux Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics. 10(9):1117. https://doi.org/10.3390/antibiotics10091117
Dawson, R. J., Locher, K. P. (2007) Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 581(5):935-8. https://doi.org/10.1016/j.febslet.2007.01.073
Du, D., van Veen, H. W., Murakami, S., Pos, K. M., Luisi, B. F. (2015) Structure, mechanism and cooperation of bacterial multidrug transporters. Curr Opin Struct Biol. 33:76-91. https://doi.org/10.1016/j.sbi.2015.07.015
Du, D., Wang-Kan, X., Neuberger, A., van Veen, H. W., Pos, K. M., Piddock, L. J. V., and Luisi, B. F. (2018). Multidrug efflux pumps: structure, function and regulation. Nature reviews. Microbiology, 16(9), 523–539. https://doi.org/10.1038/s41579-018-0048-6
Dulanto Chiang, A., Dekker, J. P. Efflux pump-mediated resistance to new beta lactam antibiotics in multidrug-resistant gram-negative bacteria. Commun Med 4, 170 (2024). https://doi.org/10.1038/s43856-024-00591-y
Edgar, R., Bibi, E. (1997) MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J Bacteriol. 179(7):2274-80. Erratum in: J Bacteriol 1997 Sep;179(17):5654. https://doi.org/10.1128/jb.179.7.2274-2280.1997
Fritz, G., Dintner, S., Treichel, N. S., Radeck, J., Gerland, U., Mascher, T., Gebhard, S. (2015). A New Way of Sensing: Need-Based Activation of Antibiotic Resistance by a Flux-Sensing Mechanism. mBio, 6(4), e00975. https://doi.org/10.1128/mBio.00975-15
Gaurav, A., Bakht, P., Saini, M., Pandey, S., Pathania, R. (2023) Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology (Reading). 169(5):001333. https://doi.org/10.1099/mic.0.001333
Gottschalk, K. E., Soskine, M., Schuldiner, S., Kessler, H. (2004) A structural model of EmrE, a multi-drug transporter from Escherichia coli. Biophys J. 86(6):3335-48. https://doi.org/10.1529/biophysj.103.034546
He, X., Szewczyk, P., Karyakin, A., Evin, M., Hong, W. X., Zhang, Q., Chang, G. (2010) Structure of a cation-bound multidrug and toxic compound extrusion transporter. Nature. 467(7318):991-4. https://doi.org/10.1038/nature09408
Heng, J., Zhao, Y., Liu, M., Liu, Y., Fan, J., Wang, X., Zhao, Y., Zhang, X. C. (2015) Substrate-bound structure of the E. coli multidrug resistance transporter MdfA. Cell Res. 25(9):1060-73. https://doi.org/10.1038/cr.2015.94
Hernando-Amado, S., Blanco, P., Alcalde-Rico, M., Corona, F., Reales-Calderón, J. A., Sánchez, M. B., Martínez, J. L. (2016) Multidrug efflux pumps as main players in intrinsic and acquired resistance to antimicrobials. Drug Resist Updat. 28:13-27. https://doi.org/10.1016/j.drup.2016.06.007
Kermani, A. A., Macdonald, C. B., Burata, O. E., Ben Koff. B., Koide, A., Denbaum, E., Koide, S., Stockbridge, R.B. (2020) The structural basis of promiscuity in small multidrug resistance transporters. Nat Commun. 11(1):6064. https://doi.org/10.1038/s41467-020-19820-8
Li, J., Her, A. S., Besch, A., Ramirez-Cordero, B., Crames, M., Banigan, J. R., Mueller, C., Marsiglia, W. M., Zhang, Y., Traaseth, N. J. (2024) Dynamics underlie the drug recognition mechanism by the efflux transporter EmrE. Nat Commun. 15(1):4537. https://doi.org/10.1038/s41467-024-48803-2
Lu, M., Radchenko, M., Symersky, J., Nie, R., Guo, Y. (2013) Structural insights into H+-coupled multidrug extrusion by a MATE transporter. Nat Struct Mol Biol. 2013 Nov;20(11):1310-7. https://doi.org/10.1038/nsmb.2687
Munita, J. M., Arias, C. A. (2016). Mechanisms of Antibiotic Resistance. Virulence Mechanisms of Bacterial Pathogens, Fifth Edition,481-511. https://doi.org/10.1128/microbiolspec.vmbf-0016-2015
Radchenko, M., Symersky, J., Nie, R., Lu, M. (2015) Structural basis for the blockade of MATE multidrug efflux pumps. Nat Commun. 6:7995. https://doi.org/10.1038/ncomms8995
Raturi, S., Nair, A.V., Shinoda, K., Singh, H., Bai, B., Murakami, S., Fujitani, H., van Veen, H. W. (2021) Engineered MATE multidrug transporters reveal two functionally distinct ion-coupling pathways in NorM from Vibrio cholerae. Commun Biol 4, 558 https://doi.org/10.1038/s42003-021-02081-6
Rodrigues, L., Villellas, C., Bailo, R., Viveiros, M., Aínsa, J. A. (2013) Role of the Mmr efflux pump in drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 57(2):751-7. https://doi.org/10.1128/AAC.01482-12
Tsutsumi, K., Yonehara, R., Ishizaka-Ikeda, E., Miyazaki, N., Maeda, S., Iwasaki, K., Nakagawa, A., Yamashita, E. (2019) Structures of the wild-type MexAB-OprM tripartite pump reveal its complex formation and drug efflux mechanism. Nat Commun. 10(1):1520. https://doi.org/10.1038/s41467-019-09463-9
Zwama, M., Nishino, K. (2021) Ever-adapting RND efflux pumps in gram-negative multidrug-resistant pathogens: A race against time. Antibiotics (Basel) 10, https://doi.org/10.3390/antibiotics10070774
April 2025, Steven Arnold, Shuchismita Dutta; Reviewed by Dr. Keith Johnson
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/amr-mech/efflux-pumps