Streptomycin Resistance
Susceptibility Testing
When possible, antibacterial substances, such as streptomycin, are tested for their effectiveness against various infectious pathogens. These test results allow clinicians to choose the antibiotic likely to result in the most effective treatment of a particular bacterial infection. For instance, one such susceptibility test provides minimum inhibitory concentration (MIC) values that can then be used to identify a pathogenic bacterial strain as susceptible, intermediate, or resistant to a certain antibiotic (Table 6). A lower MIC value indicates that a lower concentration of the antibiotic is needed to inhibit the growth of bacterial pathogens, meaning that the microorganism is susceptible to the drug. Therefore, using antibiotics with lower MIC values would result in more effective treatment of an infection.
Table 6. Minimum inhibitory concentrations (MIC) that would classify the pathogenic bacterial strain as susceptible to streptomycin, intermediate, or resistant to streptomycin (FDA 2011). These concentrations may not be the latest values approved by the US FDA.
| Pathogen | MIC (µg/mL) for Susceptible (S) strains | MIC (µg/mL) for Intermediate (I) strains | MIC (µg/mL) for Resistant (R) strains |
|---|---|---|---|
| Yersinia pestis | ≤ 4 | 8 | ≥ 16 |
| Francisella tularensis | ≤ 8 | There are no Intermediate criteria | There are no Resistant criteria |
| Brucella spp. | ≤ 8 | There are no Intermediate criteria | There are no Resistant criteria |
ResistanceMechanism(s)
Streptomycin resistance occurs when the antibiotic is not able to treat the infections because the bacterial strains causing these infections have developed mechanisms to prevent the drug from functioning. These mechanisms include (CARD, ARO:0000040 ).
* Antibiotic inactivation
* Antibiotic target alteration (through mutations)
* Antibiotic efflux
Antibiotic Inactivation
The most prevalent mechanism of streptomycin resistance is the enzymatic modification of the drug. There are three different families of aminoglycoside modifying enzymes (AMEs).
Two common types of AMEs that confer resistance to streptomycin (Becker & Cooper, 2013) include:
* ATP-dependent aminoglycoside phosphotransferases (APHs) - they phosphorylate hydroxyl groups of aminoglycosides, introducing a negative charge in the molecule which reduces their ability to bind to the ribosome. The phosphorylation may occur either at the hydroxyl group on C3 in the N-methyl-L-glucosamine [APH(3")] or the hydroxyl group on C6 in the streptidine [APH(6)] (Figure 6).
* ATP-dependent aminoglycoside nucleotidyltransferases (ANTs) - they transfer an adenosine monophosphate group from ATP to hydroxyl groups of aminoglycosides which prevents their binding to the A-site. The transfer of adenosine monophosphate (AMP) group may occur either at the hydroxyl group on C3 in the N-methyl-L-glucosamine [ANT(3")] or the hydroxyl group on C6 in the streptidine [ANT(6)] (Figure 6).
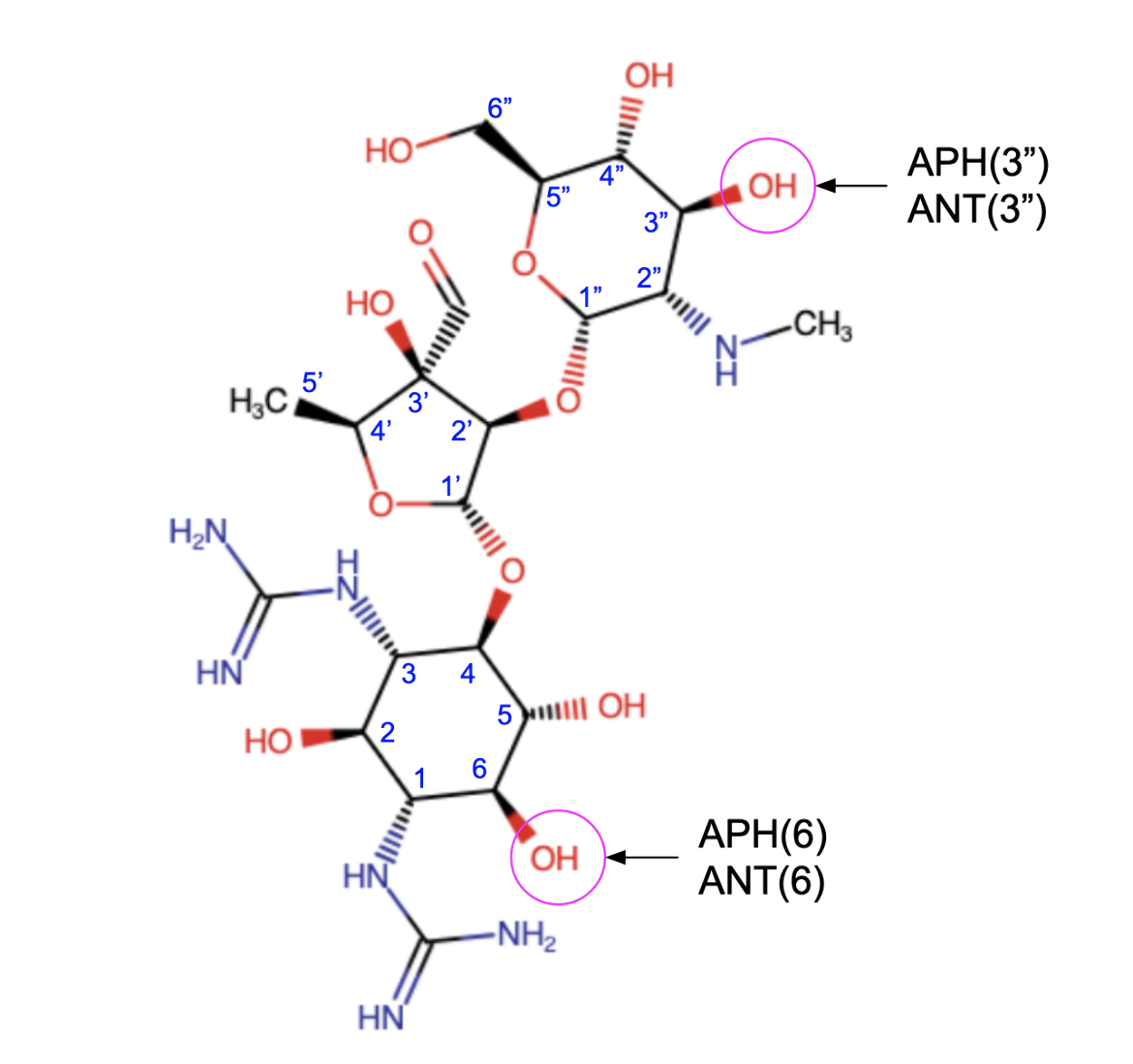
|
| Figure 6. The chemical structure of streptomycin is shown to highlight where the AMEs act. Structure created using ChemAxon. |
An example of an AME bound to streptomycin is shown here. The aminoglycoside nucleotidyltransferase, aminoglycoside (3″) adenylyltransferase or AadA, from Salmonella enterica (Figure 7) with an active-site mutant, E87Q, complexed with ATP and streptomycin shows that ATP and three rings of streptomycin are accommodated in the cleft between both domains of the enzyme (Stern et al., 2018). The Glu87 (mutated here to Gln) is the catalytic base in AadA transferring a nucleotide to the hydroxyl marked with an arrow (Figure 7). The E87Q mutant, however, is nonadenylating. Interactions of amino acids Trp173 and Asp178 are essential only for streptomycin resistance.
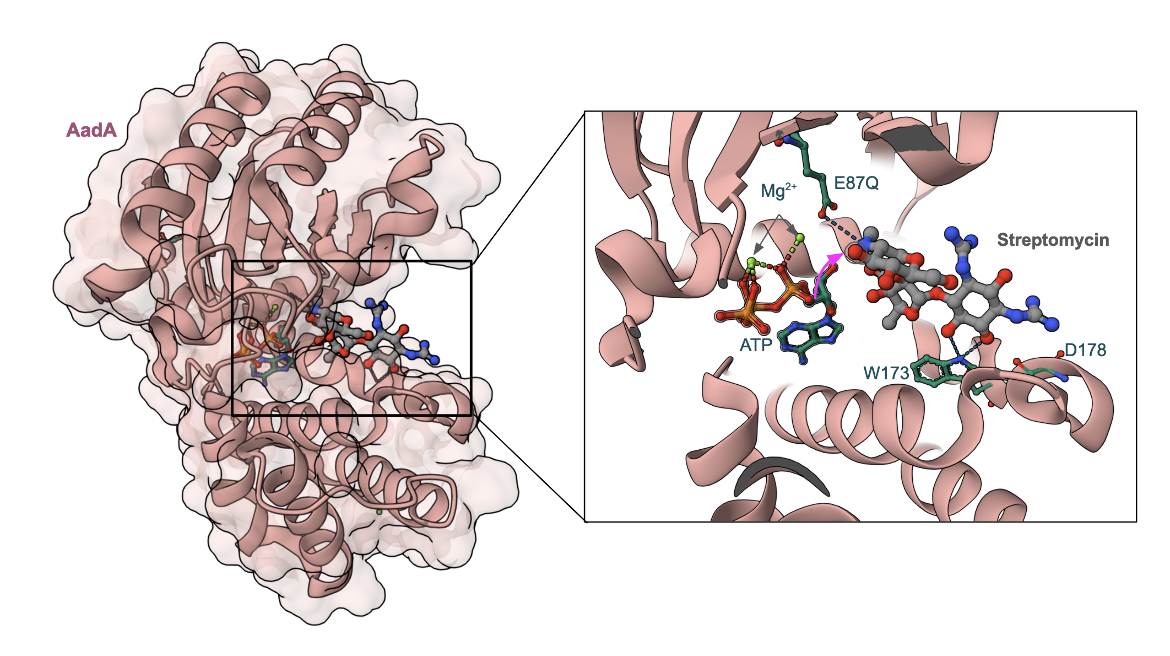
|
| Figure 7: Structure of AadA in complex with ATP, magnesium and streptomycin (PDB ID 6fzb, Stern et al., 2018) |
Learn more about aminoglycoside modifying enzymes.
Antibiotic Target Alteration – Mutations
An A1408G mutation confers high-level resistance to streptomycin as well as other aminoglycosides such as neomycin and the gentamicins (Becker & Cooper, 2013). This mutation allows the base to form a hydrogen bond with A1493 in helix 44. When streptomycin binds, the A1408G mutation is able to stabilize A1492 and A1493 which counteracts the streptomycin-induced restructuring of the decoding site (Demirci et al., 2013). Additional mutations in the 16S rRNA and S12 protein can lead to high-level streptomycin resistance, including in Mycobacterium tuberculosis (Becker & Cooper, 2013).
Antibiotic Efflux
Aminoglycosides, including streptomycin, penetrate into the cytoplasm of bacteria. However, some resistant bacterial cells quickly remove the drug through efflux pumps. As a result, the bacteria become less susceptible to the antibacterial agent. Some examples of resistance to streptomycin involving bacterial efflux pumps are described in Tale 7.
Table 7. Examples of efflux pumps that confer resistance to streptomycin.
| Cause of Resistance | Description |
|---|---|
| KpnEF | This is a small multidrug resistance (SMR) antibiotic efflux pump, originally found in Klebsiella pneumoniae. It resembles the EbrAB in E. coli and is involved in broad-spectrum antimicrobial resistance. (ARO:3004585) |
| KpnGH-TolC | This major facilitator superfamily (MFS) antibiotic efflux pump involved in drug resistance in Klebsiella pneumoniae makes bacteria resistant to a variety of antibiotics. (ARO:3004598) |
| YkkCD | This SMR antibiotic efflux pump confers resistance to cationic drugs, chloramphenicol, streptomycin and tetracycline. (ARO: 3003062) |
| AbuO | This resistance-nodulation-cell division (RND) antibiotic efflux pump found in Acinetobacter baumannii, resembles TolC and leads to multidrug resistance. (ARO:3004578) |
| LmrS | This MFS antibiotic efflux pump originally identified in Staphylococcus aureus, can lead to multidrug resistance, when expressed in E. coli. (ARO:3004572) |
Learn more about efflux pumps.
Back to the article on streptomycin.
References
Becker, B., & Cooper, M. (2012). Aminoglycoside Antibiotics in the 21st Century. ACS Chemical Biology, 8(1), 105-115. https://doi.org/10.1021/cb3005116
Demirci, H., Murphy, F., 4th, Murphy, E., Gregory, S. T., Dahlberg, A. E., & Jogl, G. (2013). A structural basis for streptomycin-induced misreading of the genetic code. Nature communications, 4, 1355. https://doi.org/10.1038/ncomms2346
Stern, A. L., Van der Verren, S. E., Kanchugal, P. S., Näsvall, J., Gutiérrez-de-Terán, H., Selmer, M. (2018) Structural mechanism of AadA, a dual-specificity aminoglycoside adenylyltransferase from Salmonella enterica. J Biol Chem. 293, 11481-11490. https://doi.org/10.1074/jbc.ra118.003989



