Gentamicin Resistance
Susceptibility Testing
When possible, antibacterial substances, such as gentamicin, are tested for their effectiveness against various infectious pathogens. These test results allow clinicians to choose the antibiotic likely to result in the most effective treatment of a particular bacterial infection. For instance, one such susceptibility test provides minimum inhibitory concentration (MIC) values that can then be used to identify a pathogenic bacterial strain as susceptible, intermediate, or resistant to a certain antibiotic (Table 6). A lower MIC value indicates that a lower concentration of the antibiotic is needed to inhibit the growth of bacterial pathogens, meaning that the microorganism is susceptible to the drug.
Table 6. Minimum inhibitory concentrations (MIC) that would classify the pathogenic bacterial strain as susceptible to (lower range number) or resistant to (higher range number) gentamicin (FDA)
| Pathogen | MIC (µg/mL) for Susceptible (S) strain | MIC (µg/mL) for Intermediate (I) strain | MIC (µg/mL) for Resistant (R) strain |
|---|---|---|---|
| Enterobacteriaceaea | ≤4 | 8 | ≥16 |
| Pseudomonas aeruginosa | ≤4 | 8 | ≥16 |
| Staphylococcus species | ≤4 | 8 | ≥16 |
Resistance Mechanism(s)
Gentamicin resistance occurs when the antibiotic is not able to treat the infections it is intended to because the bacterial strains causing these infections have developed mechanisms to prevent the drug from functioning. These mechanisms include (CARD, 2017):
* Antibiotic inactivation
* Antibiotic target alteration
* Antibiotic efflux
Antibiotic Inactivation
The most prevalent mechanism of gentamicin resistance is the enzymatic modification of the drug. There are three different families of aminoglycoside modifying enzymes (AMEs): ATP-dependent aminoglycoside phosphotransferases (APHs), ATP-dependent aminoglycoside nucleotidyltransferases (ANTs), and acetylCoA-dependent aminoglycoside acetyltransferase (AAC). The APHs phosphorylate hydroxyl groups of aminoglycosides. This adds a negative charge to the molecule and reduces its ability to bind to the ribosome. The ANTs transfer an adenosine monophosphate group from ATP to hydroxyl groups of aminoglycosides which prevents their binding to the A-site, and AACs modify amino groups.
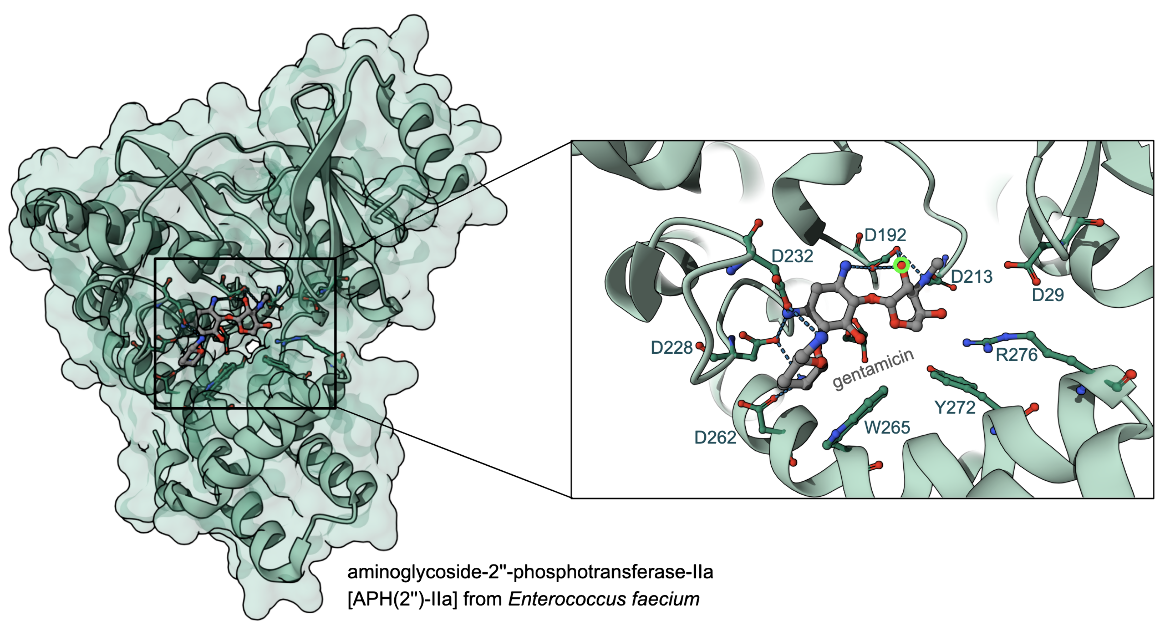
The structure of aminoglycoside-2''-phosphotransferase-IIa [APH(2'')-IIa] in complex with gentamicin in Figure 4, shows the catalytic Asp 192 (PDB ID 3ham, Young et al., 2009), interacting with and orienting the bound antibiotic, close to the site of modification (green circle in Figure 4).
Interestingly, many of the aminoglycoside modifying enzymes (AMEs) have similar substrate specificities. The crystal structures of aminoglycoside nucleotidyl transferases, ANT(2″) in complex with tobramycin or gentamicin and a nonhydrolyzable analog of ATP has helped understand the mechanism of action of these AMEs (Bassenden et al., 2016). In turn, this knowledge is likely to help in designing the next generation aminoglycoside.
Learn more about AMEs.
Antibiotic Target Alteration
The ribosome (target) may be altered either by mutations or chemical modifications. Examples of these types of target alterations are included in Tables 7 (mutations) and 8 (chemical modifications).
Table 7. Examples of mutation in 16S rRNA leading to resistance to gentamicin C (ARO:0000014)
| Cause of Resistance | Description |
|---|---|
| mutation in the 3' minor domain of helix 44 | in the rrsB 16S rRNA gene of Escherichia coli (ARO:3003396) |
| mutation in the 3' minor domain | in Borreliella burgdorferi (ARO:3003504) |
| mutation in 16S rRNA | in Mycobacteroides abscessus (ARO:3003240) |
| mutation in 16S rRNA | in Mycobacteroides chelonae (ARO:3003517) |
Table 8. Examples of enzymes that can catalyze chemical modifications of the 16S rRNA leading to resistance to gentamicin C (ARO:0000014)
| Cause of Resistance | Descriptions |
|---|---|
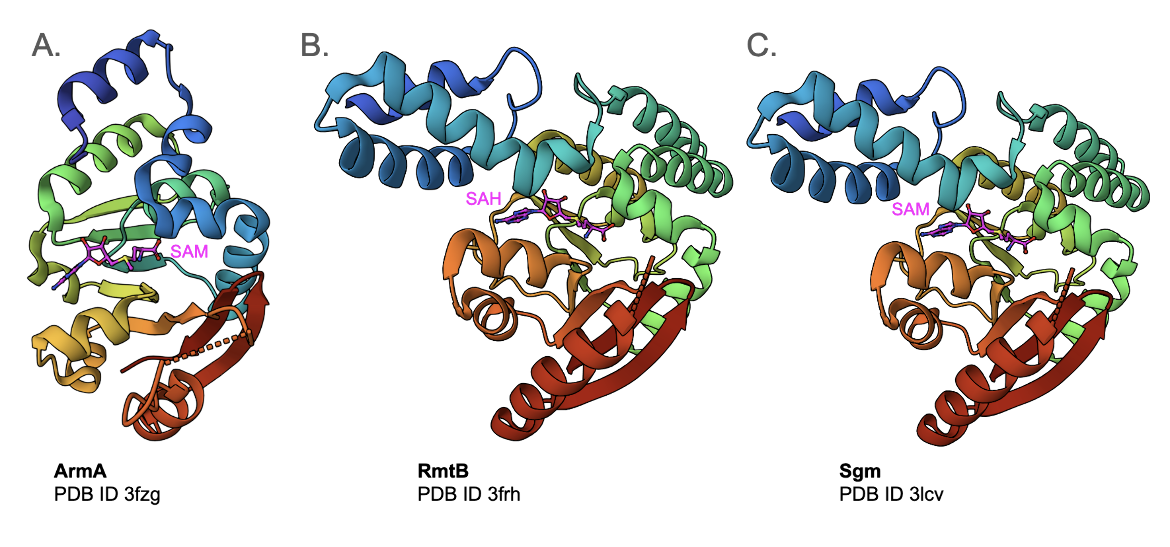
| ArmA | This is a methyltransferase that transfers a methyl group from S-adenosyl-L-methionine (SAM) to N7-G1405 of the 16S rRNA, an aminoglycoside binding siteability of gentamicin binding to its target. (ARO:3000858) The enzyme may be found in a variety of bacteria. The structure of E. coli ArmA with the cofactor SAM bound to it is shown in Figure 5A (PDB ID 3fzg, Schmitt et al., 2009). |
| RmtA and RmtB | These 16S rRNA methyltransferases found in Pseudomonas aeruginosa and other bacteria also methylates G1405. It confers high-level resistance to many aminoglycosides ARO:3000859, 3000860). The structure of E. coli RmtB with the cofactor SAM bound to it is shown in Figure 5B (PDB ID 3frh, Schmitt et al., 2009). |
| RmtC | This methyltransferase, found in Proteus mirabilis, Salmonella enterica ser. Virchow, is hypothesized to also methylate G1405 in 16S rRNA. (ARO: 3000861) |
| Sgm, or sisomicin-gentamicin methyltransferase | This enzyme methylates G1405 of 16S rRNA to confer resistance to various aminoglycosides. (ARO:3000862). The structure of Sgm from Micromonospora zionensis is shown in Figure 5C (PDB ID 3lcv, Husain et al., 2010) |
Learn more about other 16S rRNA methyltransferases.
Antibiotic Efflux
Aminoglycosides, including gentamicin, penetrate into the cytoplasm of bacteria. However, some resistant bacterial cells quickly remove the drug through efflux pumps. As a result, the bacteria become less susceptible to the antibacterial agent (Table 9). Learn more about efflux pumps.
Table 9. Examples of efflux pumps that confer resistance to streptomycin (CARD, 2017).
| Cause of Resistance | Description |
|---|---|
| KpnGH-TolC | This major facilitator superfamily (MFS) antibiotic efflux pump involved in drug resistance in Klebsiella pneumoniae makes bacteria resistant to a variety of antibiotics. (ARO:3004598) |
| MexXY-OprM | This resistance-nodulation-cell division (RND) antibiotic efflux pump expressed in Pseudomonas aeruginosa leads to multidrug resistance (ARO:3003032) |
| MexXY-OprA | This RND antibiotic efflux pump is expressed in Pseudomonas aeruginosa leading to multidrug resistance, especially to aminoglycosides. (ARO:3003038) |
| MexCD-OprJ | This RND antibiotic efflux pump with Type B NfxB mutations is more resistant to specific antibiotics such as tetracycline, chloramphenicol, and many others. (ARO:3004062) |
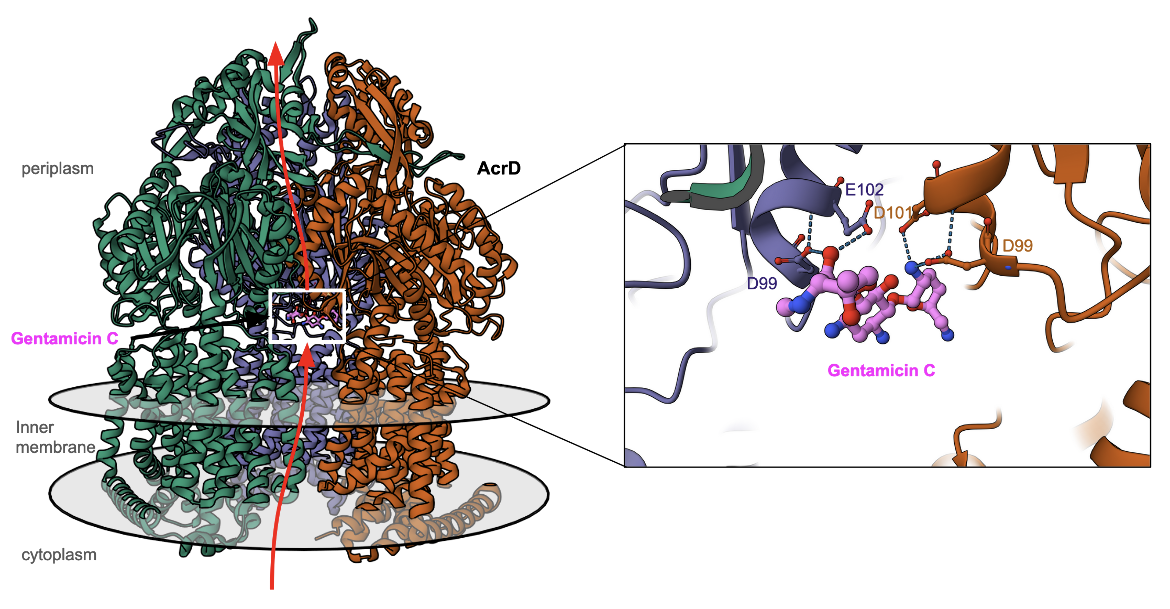
| acriflavine resistance protein D (AcrD) | This is an RND superfamily efflux pump that confers resistance to several types of antibiotic resistance in Escherichia coli. An example of AcrD bound to gentamycin in the process of being pumped out of the cytoplasm is shown in Figure 6 (Zhang et al., 2023). |
|
| Figure 6. Structure of efflux pump AcrD with gentamycin bound to it. The inset shows the interactions of gentamycin C (colored pink) and the AcrD protein. (PDB ID 8f4r, Zhang et al., 2023). |
Learn more about other efflux pumps leading to antimicrobial resistance.
Back to the article on gentamycin.
References
Bassenden, A. V., Rodionov. D., Shi, K., Berghuis, A. M. (2016) Structural Analysis of the Tobramycin and Gentamicin Clinical Resistome Reveals Limitations for Next-generation Aminoglycoside Design. ACS Chem Biol. 11(5):1339-46. https://doi.org/10.1021/acschembio.5b01070
Husain, N., Tkaczuk, K. L., Tulsidas, S. R., Kaminska, K. H., Cubrilo, S., Maravić-Vlahovicek, G., Bujnicki, J. M., Sivaraman, J. (2010) Structural basis for the methylation of G1405 in 16S rRNA by aminoglycoside resistance methyltransferase Sgm from an antibiotic producer: a diversity of active sites in m7G methyltransferases. Nucleic Acids Res. 38, 4120-32. https://doi.org/10.1093/nar/gkq122
Schmitt, E., Galimand, M., Panvert, M., Courvalin, P., Mechulam, Y. (2009) Structural bases for 16 S rRNA methylation catalyzed by ArmA and RmtB methyltransferases. J Mol Biol., 388, 570-82. https://doi.org/10.1016/j.jmb.2009.03.034
Young, P. G., Walanj, R., Lakshmi, V., Byrnes, L. J., Metcalf, P., Baker, E. N., Vakulenko, S. B., Smith, C. A. (2009) The crystal structures of substrate and nucleotide complexes of Enterococcus faecium aminoglycoside-2''-phosphotransferase-IIa [APH(2'')-IIa] provide insights into substrate selectivity in the APH(2'') subfamily. J Bacteriol. 191(13):4133-43. https://doi.org/10.1128/jb.00149-09
Zhang, Z., Morgan, C. E., Cui, M., Yu, E. W. (2023) Cryo-EM Structures of AcrD Illuminate a Mechanism for Capturing Aminoglycosides from Its Central Cavity. mBio., 14, e0338322. https://doi.org/10.1128/mbio.03383-22