Ribosomal Methyltransferases
Introduction
The ribosome is a complex machine responsible for protein synthesis in all cells. It is comprised of RNA and various proteins that work together to function as the site of protein synthesis. Several classes of antibiotics target specific locations of the ribosomal RNA (rRNA) to block protein synthesis in bacteria. Learn more about ribosomes. In general, RNA undergoes several types of post-transcriptional modifications to increase the diversity of its composition and facilitate its functions (Lopez Sanchez et al., 2020). Although more common in tRNAs, many modifications (including methylations) are common in rRNAs too.
Normal function of ribosomal methyltransferases
The precise role of all rRNA modifications is not yet fully understood. However, many of these modifications occur on highly conserved nucleotides, located in functionally important parts, so they are likely to have important roles in ribosomal function (Lopez Sanchez et al., 2020). Also, the modification sites are not all modified at the same time, suggesting that these modifications may occur under different physiological conditions and/or may have a role in the regulation of protein synthesis. For example, in bacterial ribosomes, methylation of nucleotides in the small subunit often occurs on the surface and is introduced during the late stages of ribosome assembly. On the other hand, modifications in the large subunit occur during the early stages of assembly (Lopez Sanchez et al., 2020).
A few of the rRNA methylations are conserved and facilitate ribosomal function. For example,
* In the E. coli 16S rRNA, the ksgA methyltransferase modifies the bases at positions A1518 and A1519 to facilitate contacts near the decoding center of the ribosome and stabilize this site (Formenoy et al., 1994).
* In the E. coli 23S rRNA, methylation at G2251 and U2552, introduced by the methyltransferases RlmB and RlmE, respectively helps accommodate the aminoacyl-tRNA in the ribosome (Lövgren and Wikström, 2001).
rRNA methyltransferases in Antibiotic Resistance
Two classes of methyltransferases have been reported to cause resistance to antibiotics:
* 16S ribosomal RNA methyltransferase
* 23S ribosomal RNA methyltransferase
16S ribosomal RNA methyltransferase
In many aminoglycoside-producing bacteria, e.g., Streptomyces and Micromonospora species, posttranslational methylation of the 16S rRNA confers resistance to multiple aminoglycosides (Kawai et al., 2021). Two types of methylation activities have been reported:
* methylation of the N1 position of A1408 in 16 S rRNA - this confers resistance to kanamycin and gentamicin groups of aminoglycosides, neomycin group, and apramycin. Examples of G1408 16S rRNA methyltransferase include the NpmA family of enzymes (NpmA1 in Escherichia coli and NpmA2 in Clostridioides difficile) (Kawai et al., 2021).
* methylation of the N7 position of G1405 in 16S rRNA - this confers resistance to only 4,6-disubstituted 2-deoxystreptamines. Examples of G1405 16S rRNA methyltransferase include ArmA, and the ribosomal RNA methyltransferase family of enzymes (RmtA, RmtB, RmtC12, RmtD)
NpmA methyltransferase
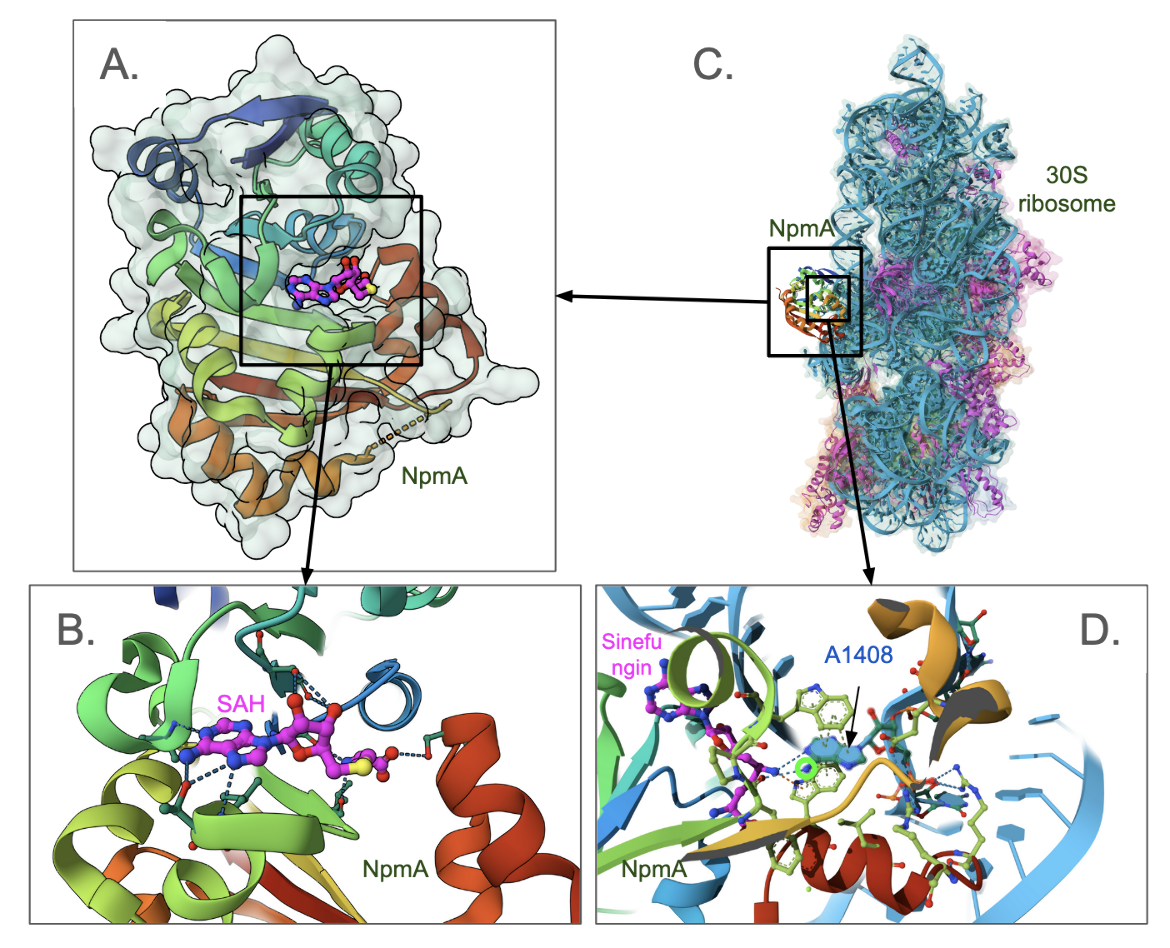
NpmA (novel plasmid-mediated aminoglycoside resistance rRNA methyltransferase A) is a methyltransferase enzyme, responsible for modifying the 16S target binding site, at the N1 position of residue A1408. It makes bacteria resistant to the 4,6- and 4,5-di-substituted aminoglycosides through the process of methylation at the N1 position of nucleotide A1408 (Krause, 2016). In other words, the antibiotic’s target is to disrupt protein translation within the 30S ribosomal subunit, however, the prevailing resistance mechanism is modification of this target by enzymatic methylation.
NpmA was discovered from E. coli clinical strain ARS3, as the first methyltransferase that can methylate 16S rRNA at A1408. It is part of the Kam family of methyltransferases, causing resistance against aminoglycosides. Interestingly, it also shares high structural similarity to TrmB, in Bacillus subtilis, part of the TrmB/Trm8 family (Husain et al., 2010). The Kam family (e.g., KamA and KamB) also encompasses enzymes that methylate at the same position, but in antibiotic producers. However, NpmA is derived from the Escherichia coli clinical strain ARS3 and is the first methyltransferase found in a clinical isolate. The npmA gene is found on a bacterial plasmid, indicating horizontal gene transfer would easily allow for the efficient spread and swift global emergence of this resistance mechanism.
The NpmA enzyme is a Class I methyltransferase, composed of a seven+ stranded β-sheet, flanked by α-helices on each side forming an αβα-sandwich (Figure 1A). The enzyme has The SAH (SAM cofactor after it has donated the methyl group) bound to it (Figure 1B). The carbon atoms of SAH are colored magenta. Another structure shows how NpmA binds to the small subunit of the ribosome (PDB ID 4ox9, Dunkle et al., 2014, Figure 1C.) shown in the molecule. This structure has a SAM analog bound to the position where SAM binds positioned close to the N1 in the A1408 for methylation (Figure 1D).
RmtC methyltransferase
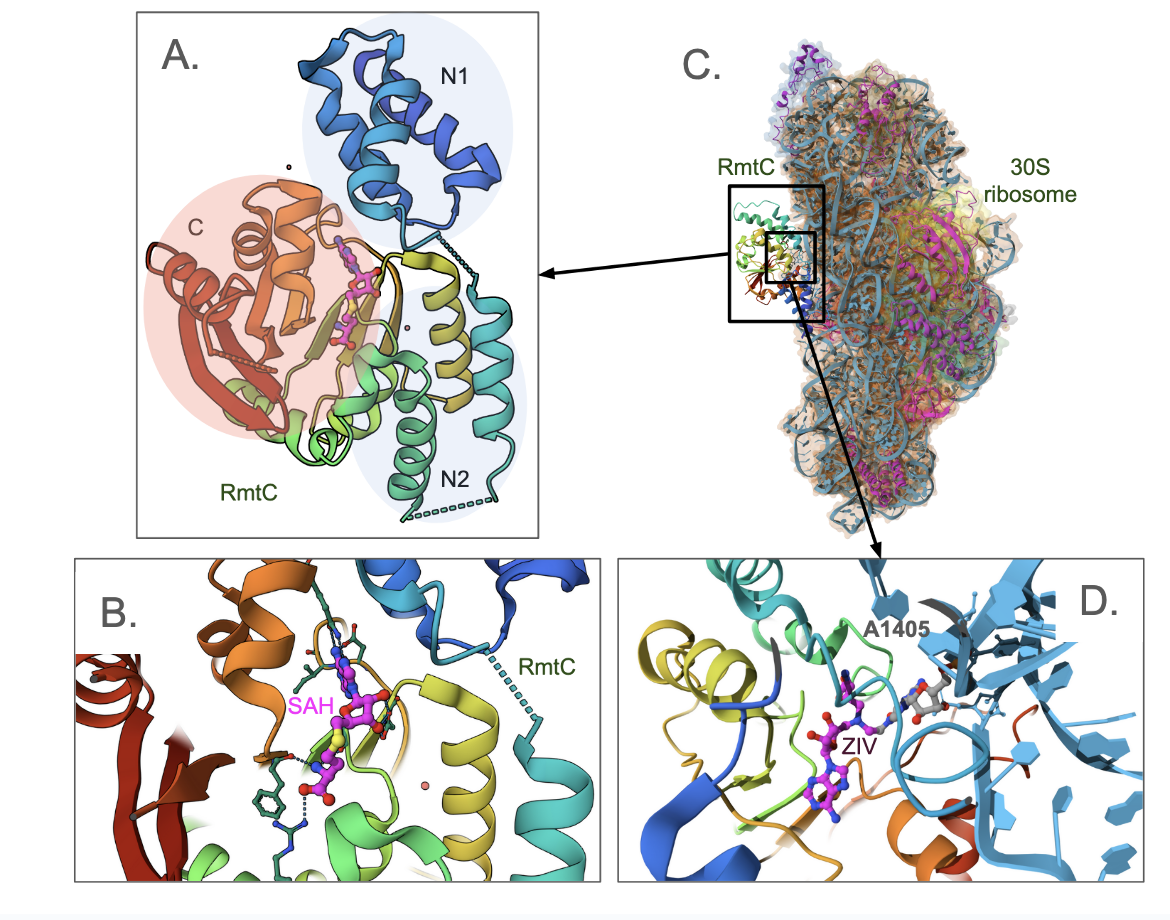
The ribosomal RNA methyltransferase (Rmt) C methylates 16S rRNA on N7 of G1405. This enzyme has been found in bacteria that produce aminoglycoside. This enzyme has been found in bacteria that produce aminoglycoside and in clinically drug-resistant pathogenic ones (PDB ID 6pqb, Nosrati et al., 2019). The crystal structure of the protein shows that it has three domains - two helical domains in the N-terminus (labeled N1 and N2 in Figure 2A) and a C-terminal Rossmann fold protein, seen in canonical class I methyltransferase. The N1 helices form a helical bundle, while the N2 helices extend the helices on one face of the αβα-sandwich. The SAM/SAH binds to the C-terminal domain. A closeup of the SAH binding site includes amino acid residues from all three domains (Figure 2B).
The structure of RmtC bounds to the small subunit of the ribosome (PDB ID 8ghu, Srinivas et al., 2023, Figure 2C.) shown in the molecule. Like in the structure of NpmA bound to the 30S ribosome (Figure 1C), here too the G1405 base is flipped out for methylation. In this structure, an analog of SAM is covalently linked to the N7 position of G1405. This artificial construct was used to trap the 30S–RmtC complex in a post-catalytic state. In a closeup of the structure (Figure 2D) the SAH part of the structure is shown such that the carbon atoms are colored magenta, while the G1405 part of the molecule has grey colored carbon atoms.
In gram-negative bacteria, acquired 16S rRNA methyltransferases ArmA and NpmA confer resistance to clinically used aminoglycosides by modifying the A-site (Lioy et al., 2014).
The ArmA and Rmt family of methyltransferases have been identified in 3% to 27% of aminoglycoside-resistant gram-negative infections in hospitals worldwide (Srinivas et al., 2023). With horizontal gene transfer these resistance genes are likely to spread rapidly leading to further challenges in treating infections.
Learn about examples of 16S rRNA methylation leading to gentamicin C resistance.
23S ribosomal RNA methyltransferase
Two specific classes of methyltransferases are discussed herein:
* Erm family of methyltransferases (specifically ErmE) - that methylates A2508
* Cfr family of methyltransferase - that methylate A2503.
ErmE methyltransferase
Over 70 years ago the role of rRNA methyltransferases in causing erythromycin resistance was discovered. Erythromycin resistance rRNA methyltransferases (Erm family of methyltransferases) confer resistance to macrolide, lincosamide, and streptogramin B drugs (MLSB resistance phenotype) (Leclercq and Courvalin, 1991). The resistance is caused by methylation of a number of different bases in the 23S rRNA - including G748, A1067, C1920, A2058, G2470, U2479, A2503 and G2535 (Vester and Long, 2013).
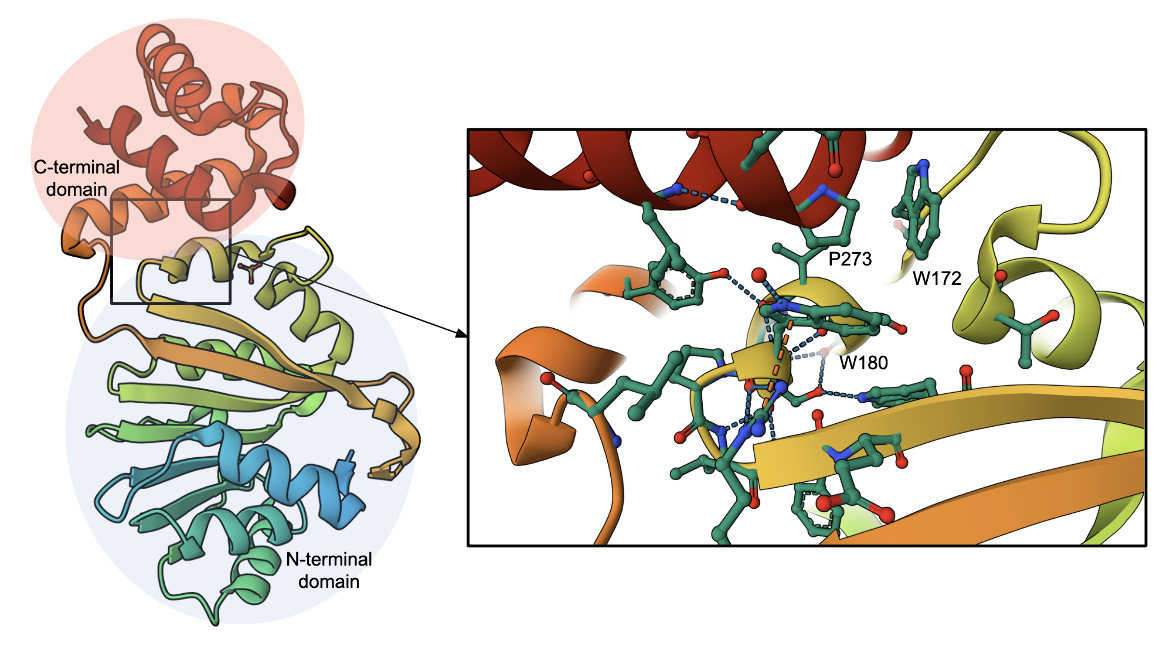
The ErmE methyltransferase from Saccharopolyspora erythraea dimethylates A2058 in 23S rRNA, where S-adenosyl methionine (SAM) functions as the methyl donor. Methylation at this site protects the ribosome from binding macrolides, leading to resistance. The enzyme has two main domains - an N-terminal Rossmann-like α/ß catalytic domain and a C-terminal helical domain (Figure 3, left, Stsiapanava and Selmer, 2019). The cofactor SAM binds to the N-terminal domain. The SAM binding and catalytic residues are highly conserved in all members of this family (including ErmC', Erm38). The C-terminal domain and its connectors may vary in these enzymes. A closeup of the interactions between the N- and C-domains is mediated by three amino acids Pro273, Trp172, and Trp180 (Figure 3 inset).
The erm genes have been shown to be transmitted both horizontally and vertically (Goh et al., 2022). While horizontal transfers are seen in methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci, the endogenous and inducible Erms in Mycobacterium, limit the utility of macrolides, lincosamides, and streptogramins (MLS) antibiotics.
|
| Figure 3. Structure of 23S rRNA methyltransferase ErmE (PDB ID 6nvm, Stsiapanava and Selmer, 2019). The inset shows the interactions amongst amino acids at the interface of the N- and C-termini. |
Cfr methyltransferases
In 2008, a dozen patients in the intensive care unit of a hospital in Spain and 3 other patients were found to be infected with methicillin-resistant Staphylococcus aureus that was also resistant to linezolid (Morales et al., 2010). A new mechanism of linezolid resistance was reported in veterinary staphylococcal isolates due to the acquisition of the chloramphenicol-florfenicol resistance (cfr) gene. This gene is primarily found in plasmids and appears to be capable of horizontal transfer between staphylococci. The product of the cfr gene is a methyltransferase that catalyzes methylation of A2503 in the 23S rRNA of the large ribosomal subunit. This modification confers resistance to chloramphenicol, florfenicol, and clindamycin.
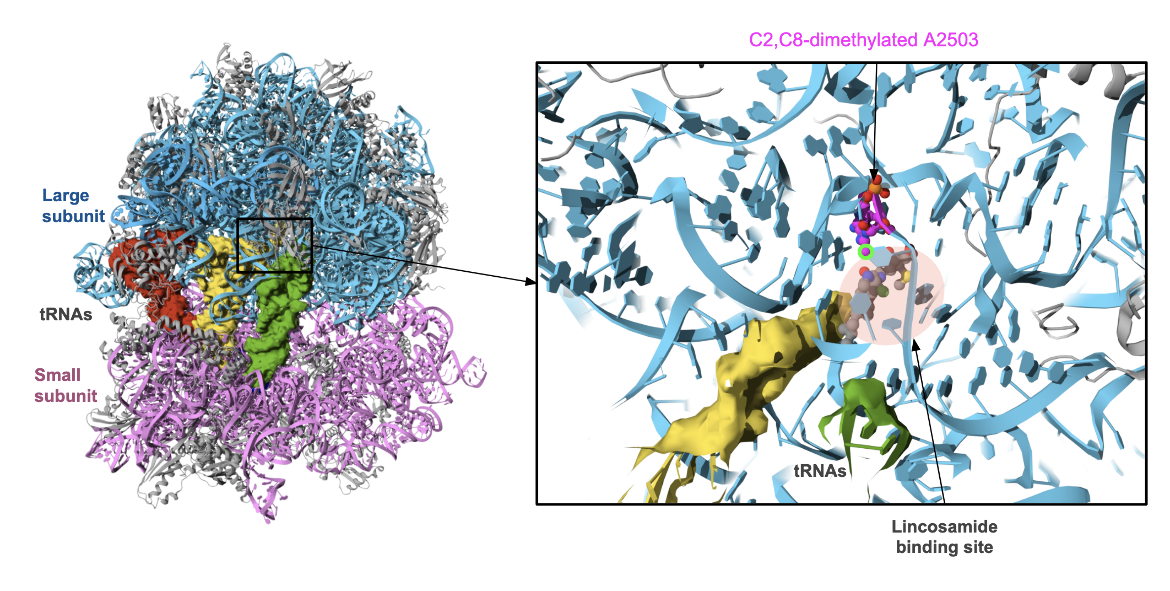
Although structures of the Cfr methylases have not been experimentally determined, the impact of methylating A2503 has been studied. A series of crystal structures of a di-methylated A2503-containing Thermus thermophilus 70S ribosome were determined (Aleksandrova et al., 2024). Close examination of these structures show that the methylation of A2503 at the C8 position by Cfr methylases (marked with a fluorescent green halo in Figure 4, right) sterically clashes with the lincosamide binding site (marked with a red-shaded oval in the same figure). Thus when A2503 is methylated at the C8 position, the antibiotics that normally bind at the peptidyl transferase center can no longer bind. As a result, the ribosome continues with protein synthesis. Interestingly, a synthetic lincosamide, called iboxamycin is shown to be slightly different from other lincosamides - it introduces a slight shift in the location of A2503 avoiding steric clashes with the methylated base (A2503).
Clinical Implications of rRNA methylation
Methylation of the 16S rRNA (at positions A1408 and G1405) leads to aminoglycoside resistance. Horizontal transfer of 16S rRNA methylase gene from some aminoglycoside-producing microorganisms to P. aeruginosa suggests that such spread, especially in hospitals and clinical settings can be a matter of concern (Yokoyama et al., 2003). Methylation of 23S rRNA (e.g., at A2058 by Erm family of methyltransferases and at A2503 by Cfr enzymes) leads to macrolide, lincosamide, and streptogramin resistance (Leclercq and Courvalin, 1991). Other methyltransferases that act at 30S rRNA positions G748, A1067, and more have also been studied.
Better understanding the specific interactions of the methylated bases and various antibiotics that bind the ribosome can help us design molecules that can bypass resistance mechanisms and restore inhibition by antibiotics.
References
Aleksandrova, E. V., Wu, K. J. Y., Tresco, B. I. C., Syroegin, E. A., Killeavy, E. E., Balasanyants, S. M., Svetlov, M. S., Gregory, S. T., Atkinson, G. C., Myers, A. G., Polikanov, Y. S. (2024) Structural basis of Cfr-mediated antimicrobial resistance and mechanisms to evade it. Nat Chem Biol. 2024 Jul;20(7):867-876. https://doi.org/10.1038/s41589-023-01525-w
Dunkle, J. A., Vinal, K., Desai, P. M., Zelinskaya, N., Savic, M., West, D. M., Conn, G. L., Dunham, C. M. (2014) Molecular recognition and modification of the 30S ribosome by the aminoglycoside-resistance methyltransferase NpmA. Proc Natl Acad Sci U S A. 111(17):6275-80. https://doi.org/10.1073/pnas.1402789111
Formenoy, L. J., Cunningham, P. R., Nurse, K., Pleij, C. W., Ofengand, J. (1994) Methylation of the conserved A1518-A1519 in Escherichia coli 16S ribosomal RNA by the ksgA methyltransferase is influenced by methylations around the similarly conserved U1512.G1523 base pair in the 3' terminal hairpin. Biochimie. 76(12):1123-8. https://doi.org/10.1016/0300-9084(94)90040-x
Goh, B. C., Xiang, X., Lescar, J., Dedon, P. C. (2022) Crystal structure and functional analysis of mycobacterial erythromycin resistance methyltransferase Erm38 reveals its RNA-binding site. J Biol Chem. 298(2):101571. https://doi.org/10.1016/j.jbc.2022.101571
Husain, N., Obranic, S., Koscinski, L., Seetharaman, J., Babic, F., Bujnicki, J. M., Maravic-Vlahovicek, G., Sivaraman, J. (2011) Structural basis for the methylation of A1408 in 16S rRNA by a panaminoglycoside resistance methyltransferase NpmA from a clinical isolate and analysis of the NpmA interactions with the 30S ribosomal subunit. Nucleic Acids Res. 39(5):1903-18. https://doi.org/10.1093/nar/gkq1033
Kawai, A., Suzuki, M., Tsukamoto, K., Minato, Y., Doi, Y. (2021) Functional and Structural Characterization of Acquired 16S rRNA Methyltransferase NpmB1 Conferring Pan-Aminoglycoside Resistance. Antimicrob Agents Chemother. 65(10):e0100921. https://doi.org/10.1128/aac.01009-21
Krause, K. M., Serio, A. W., Kane, T. R., Connolly, L. E. (2016) Aminoglycosides: An Overview. Cold Spring Harb Perspect Med. 6(6):a027029. https://doi.org/10.1101/cshperspect.a027029
Leclercq, R., Courvalin, P. (1991) Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 35(7):1267-72. https://doi.org/10.1128/aac.35.7.1267
Lioy, V. S., Goussard, S., Guerineau, V., Yoon, E. J., Courvalin, P., Galimand, M., Grillot-Courvalin, C. (2014) Aminoglycoside resistance 16S rRNA methyltransferases block endogenous methylation, affect translation efficiency and fitness of the host. RNA. 20(3):382-91. https://doi.org/10.1261/rna.042572.113
Lopez Sanchez, M. I. G., Cipullo, M., Gopalakrishna, S., Khawaja, A., Rorbach, J. (2020) Methylation of Ribosomal RNA: A Mitochondrial Perspective. Front Genet. 11:761. https://doi.org/10.3389/fgene.2020.00761
Lövgren, J. M., and Wikström, P. M. (2001). The rlmB gene is essential for formation of Gm2251 in 23S rRNA but not for ribosome maturation in Escherichia coli. J. Bacteriol. 183, 6957–6960. https://doi.org/10.1128/jb.183.23.6957-6960.2001
Morales, G., Picazo, J. J., Baos, E., Candel, F. J., Arribi, A., Peláez, B., Andrade, R., de la Torre, M. A., Fereres, J., Sánchez-García, M. (2010) Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin Infect Dis. 50(6):821-5. https://doi.org/10.1086/650574
Nosrati, M., Dey, D., Mehrani, A., Strassler, S. E., Zelinskaya, N., Hoffer, E. D., Stagg, S. M., Dunham, C. M., Conn, G. L. (2019) Functionally critical residues in the aminoglycoside resistance-associated methyltransferase RmtC play distinct roles in 30S substrate recognition. J Biol Chem. 294(46):17642-17653. https://doi.org/10.1074/jbc.ra119.011181
Srinivas, P., Nosrati, M., Zelinskaya, N., Dey, D., Comstock, L. R., Dunham, C. M., Conn, G. L. (2023) 30S subunit recognition and G1405 modification by the aminoglycoside-resistance 16S ribosomal RNA methyltransferase RmtC. Proc Natl Acad Sci U S A. 120(25):e2304128120. https://doi.org/10.1073/pnas.2304128120
Stsiapanava, A., Selmer, M. (2019) Crystal structure of ErmE - 23S rRNA methyltransferase in macrolide resistance. Sci Rep. 9(1):14607. https://doi.org/10.1038/s41598-019-51174-0
Vester, B., Long, K. S. (2000-2013) Antibiotic Resistance in Bacteria Caused by Modified Nucleosides in 23S Ribosomal RNA. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; Available from: https://www.ncbi.nlm.nih.gov/books/NBK6514/
Yokoyama, K., Doi, Y., Yamane, K., Kurokawa, H., Shibata, N., Shibayama, K., Yagi, T., Kato, H., Arakawa, Y. (2003) Acquisition of 16S rRNA methylase gene in Pseudomonas aeruginosa. Lancet. 362(9399):1888-93. https://doi.org/10.1016/s0140-6736(03)14959-8
April 2025, Shuchismita Dutta; Reviewed by Dr. Dr. Gerard Wright
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/amr-mech/ribo-meth