Piperacillin
Drug Name
Piperacillin is a β-lactam antibiotic used to treat bacterial infections usually caused by gram-positive organisms, though it has in vitro activity against gram-positive and gram-negative aerobic and anaerobic bacteria. It is usually taken with the β-lactamase inhibitor tazobactam to overcome bacteria that resist piperacillin via β-lactamases.
Table 1. Basic profile of piperacillin.
| Description | Intravenously administered semi-synthetic, broad-spectrum, ampicillin derived ureidopenicillin antibiotic |
| Target(s) | Penicillin-binding proteins (PBPs) |
| Generic | Piperacillin |
| Commercial Name | Pipracil, Zosyn |
| Combination Drug(s) | Zosyn® (piperacillin & tazobactam) |
| Other Synonyms | Pipercillin, Piperacillin anhydrous, Piperacillin sodium, Pipril |
| IUPAC Name | (2S,5R,6R)-6-[(2R)-2-[(4-ethyl-2,3-dioxopiperazine-1-carbonyl)amino]-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| Ligand Code in PDB | WPP, JPP (open form) |
| PDB Structure | 4kqo (PBP-3 in a covalent complex with Piperacillin) |
| ATC code | J01CA12 |
|
|
|
Antibiotic Chemistry
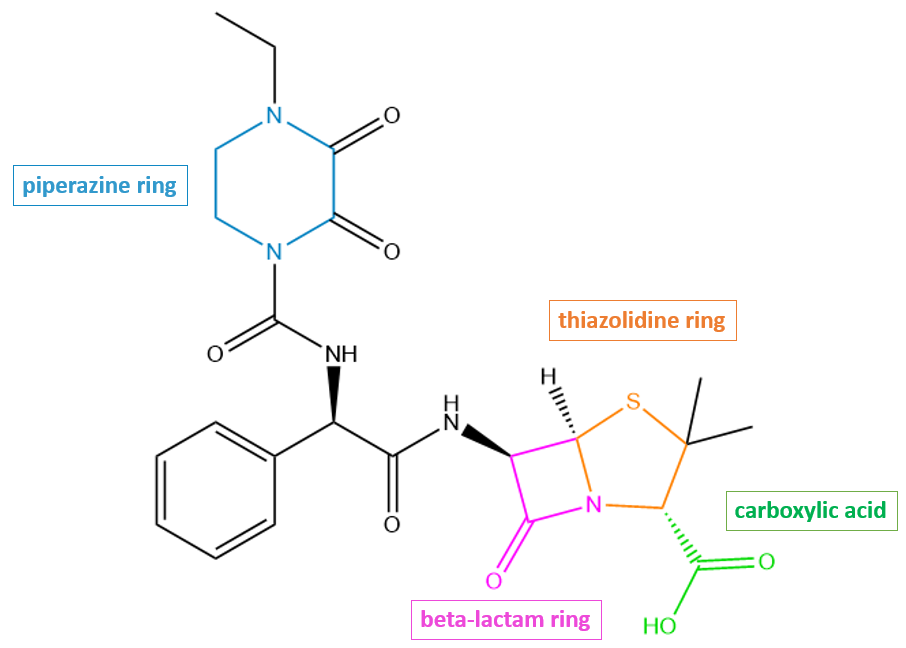
Piperacillin has the β-lactam and thiazolidine rings like any other penicillin-like antibiotic. In addition, it has a few additional structural features that contribute to its antibacterial properties. For instance, the piperazine ring moiety (shown in blue Figure 2) contributes to pi-stacking interactions that stabilize the drug in the active site of PBP3.
|
| Figure 2. 2D structure of piperacillin showing the functional moieties responsible for antibacterial activity. Structure created using ChemDraw. |
Drug Information
Table 2. Chemical and physical properties (DrugBank).
| Chemical Formula | C23H27N5O7S |
| Molecular Weight | 517.555 g/mol |
| Calculated Predicted Partition Coefficient: cLogP | 0.67 |
| Calculated Predicted Aqueous Solubility: cLogS | -3.6 |
| Solubility (in water) | 0.119 mg/mL |
| Predicted Topological Polar Surface Area (TPSA) | 156.43 Å2 |
Drug Target
Piperacillin disrupts cell wall biosynthesis in bacteria by inhibiting the penicillin-binding proteins (PBPs). This name originates from the ability of penicillin and other β-lactam antibiotics to bind to these proteins. Some of these PBP enzymes are responsible for catalyzing the final steps of the peptidoglycan synthesis pathway, which include polymerizing glycan strands and then cross-linking adjacent chains to form the characteristic mesh structure of peptidoglycan, while other PBPs are involved in regulating peptidoglycan recycling and cell wall remodeling.
Learn more about PBPs.
Inhibition of the PBPs responsible for cross-linking results in a severely weakened cell wall, which then causes bacterial cell lysis and death. The discussion here will be focused on PBP-3 in Pseudomonas aeruginosa as the target of piperacillin. PBP3 serves as a transpeptidase during peptidoglycan synthesis in P. aeruginosa cells. This means that in the absence of piperacillin, PBP3 is able to catalyze cross-linking between glycan strands to form the mesh-like structure of peptidoglycan. It is a monofunctional enzyme because it is only involved in cross-linking, or transpeptidation (not in transglycosylation, the process of polymerizing the nascent glycan strands).
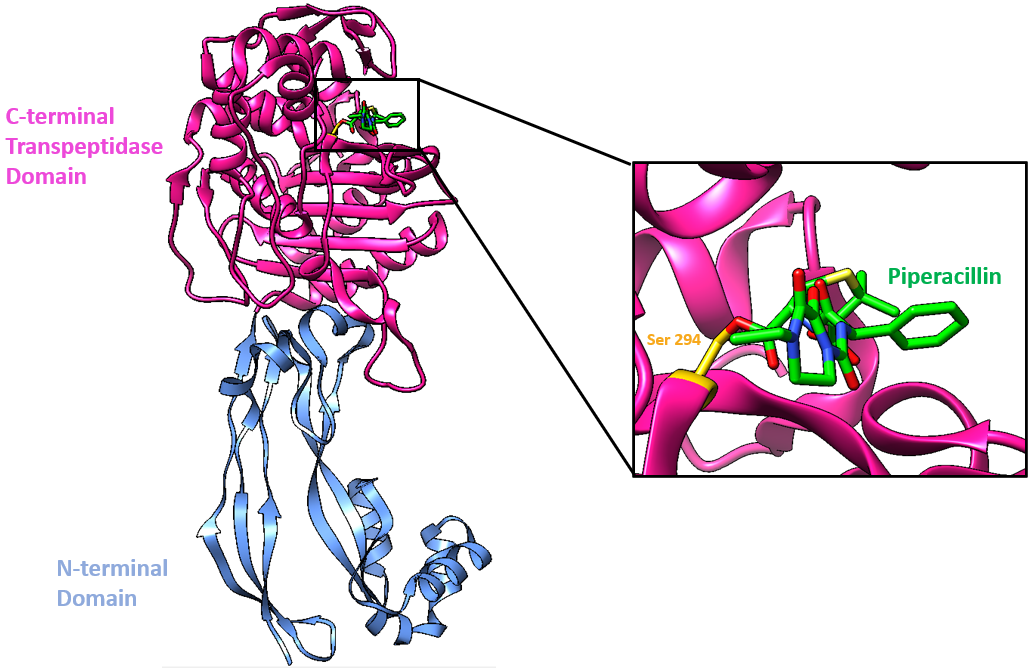
This PBP3 enzyme is made of two domains (Han et al., 2010, Figure 2):
* N-terminal domain (blue)
* Penicillin-binding domain aka C-terminal Domain (pink) where β-lactam antibiotics, such as piperacillin, bind (Figure 3).
Drug-Target Complex
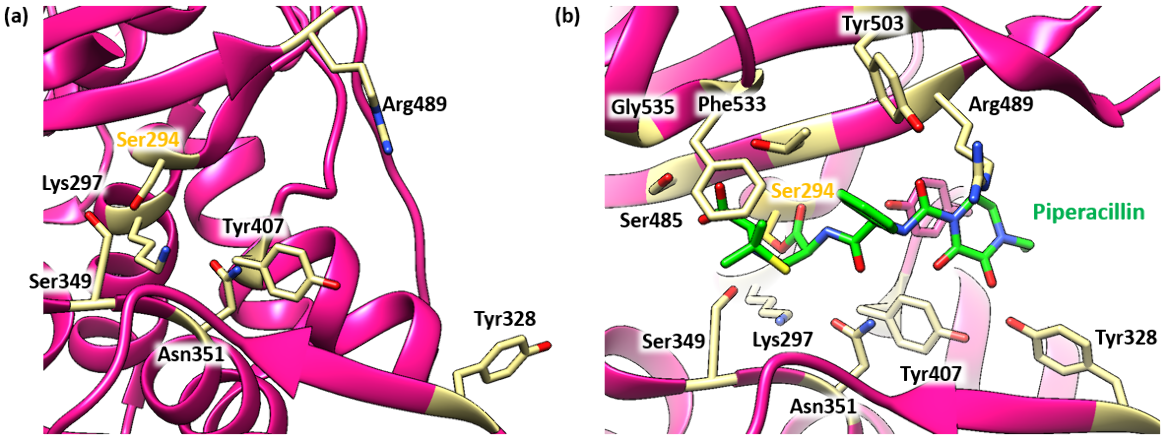
Piperacillin is able to bind to and inhibit PBP3 due to the structural similarity it shares with the terminal D-Ala-D-Ala peptide bond of the natural substrate. Specifically, the β-lactam ring, which is characteristic of all β-lactam antibiotics, is a structural mimic of the backbone of the D-Ala-D-Ala peptide, allowing the antibiotic to occupy the same binding site as the natural substrate. The Ser294 nucleophile is where the D-Ala-D-Ala peptide bond of the peptide substrate binds in the active site and takes part in the transpeptidation reaction.
The antibiotic reacts with the Ser294 nucleophile and forms an acyl-enzyme covalent complex (Figure 4). As the β-lactam blocks the Ser294 residue, the natural peptide substrate can no longer access the active site of PBP3. Thus, piperacillin inactivates the enzyme and prevents transpeptidation. The position of the ester linkage in the piperacillin-bound complex is very similar to that of other β-lactams that bind to PBP3. The ester linkage in piperacillin, along with the carbonyl oxygen of the β-lactam ring, form hydrogen bonds with the main chain NHs of the Thr487 (2.9 Å) and Ser294 (2.8 Å) residues in PBP3. This forms the oxyanion hole. As in other β-lactam bound PBP3 structures (see aztreonam, ceftazidime, amoxicillin, and meropenem), the C-3 carboxylate is positioned to interact with Ser485 (2.2 Å, Oγ), Lys484 (3.8 Å, Nε) and Thr487 (2.7 Å, Oγ). The lengths of the hydrogen bonds and the atom in the PBP3 with which the drug interacts are included in parentheses.
In the piperacillin-bound structure, the nucleophilic Ser294 side chain is positioned to hydrogen bond with the backbone carbonyl oxygen of Ser485. On the other hand, in the apo-PBP3 structure, the nucleophilic Ser294 hydroxyl is positioned between Lys297 (2.7 Å) and Lys484 (2.8 Å).
Pharmacologic Properties and Safety
Table 3. Pharmacokinetics: ADMET of piperacillin.
| Features | Comment(s) | Source |
|---|---|---|
| Absorption | Not absorbed following oral administration | DrugBank |
| IC50 | 16 μg/mL (for binding to PBP1a, PBP1b of K. pneumoniae); 2 μg/mL (for binding to PBP2 of K. pneumoniae); 0.06 μg/mL (for binding to PBP3 of K. pneumoniae); 64 μg/mL (for binding to PBP4, PBP5, PBP6 of K. pneumoniae); 0.056 μg/mL (for binding to PBP3 of S. pneumoniae) | (Sutaria et al., 2018) |
| Ki (µM) | N/A | N/A |
| Half-life (hrs) | ~ 0.5 - 1.5 hours | DrugBank |
| Duration of Action | 8-12 hours | FDA |
| Absorption Site | N/A | N/A |
| Transporter(s) | N/A | N/A |
| Metabolism | Largely not metabolized | DrugBank |
| Excretion | Excreted by the biliary (liver) route and renal route. Therefore, it can be used in patients with severely restricted kidney function. | DrugBank |
| AMES Test (Carcinogenic Effect) | Probability 0.9133 (Non AMES toxic) | DrugBank |
| hERG Safety Test (Cardiac Effect) | Probability 0.9902 (weak inhibitor) | DrugBank |
| Liver Toxicity | Piperacillin has been linked with idiosyncratic liver injury but is rare. Liver injury can be severe but is usually self-resolved once piperacillin treatment is stopped. | LiverTox |
Drug Interactions and Side Effects
Table 4. Drug interactions and side effects of piperacillin.
| Features | Comment(s) | Source |
|---|---|---|
| Total Number of Drug Interactions | 51 drugs | Drugs.com |
| Major Drug Interaction(s) | bcg; cholera vaccine, live; methotrexate; typhoid vaccine, live; vancomycin | Drugs.com |
| Alcohol/Food Interaction(s) | Diets with sodium restriction for patients with heart failure, hypertension, and fluid retention. Clinical monitoring of electrolytes is recommended when administering piperacillin for these patients. | Drugs.com |
| Disease Interaction(s) | Colostridioides difficile associated diarrhea (CDAD) (major); Coagulation abnormalities (moderate); Renal dysfunction (moderate); Hemodialysis (moderate); Cystic fibrosis (moderate); Seizures (moderate); Hypokalemia, congestive heart failure, fluid retention, hypertension, hypernatremia (moderate) | Drugs.com |
| On-target Side Effects | Irritation at injection site | Drugs.com |
| Off-target Side Effects | Rash, hives, itching, fevers or chills, sore throat, seizures, extra muscle action, diarrhea | Drugs.com |
| CYP Interactions | CYP450 3A4 substrate | DrugBank |
Links
Table 5. Links to learn more about piperacillin
| Comprehensive Antibiotic Resistance Database (CARD) | ARO: 0000078 |
| DrugBank | DB00319 |
| Drugs.com | https://www.drugs.com/cdi/piperacillin.html |
| FDA | https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050684s88s89s90_050750s37s38s39lbl.pdf |
| LiverTox: National Institutes of Health (NIH) | https://www.ncbi.nlm.nih.gov/books/NBK548463/ |
| PubChem CID | 43672 |
Learn about piperacillin resistance.
References
Han, S., Zaniewski, R. P., Marr, E. S., Lacey, B. M., Tomaras, A. P., Evdokimov, A., Miller, J. R., Shanmugasundaram, V. (2010) Structural basis for effectiveness of siderophore-conjugated monocarbams against clinically relevant strains of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A., 107, 22002-7. https://doi.org/10.1073/pnas.1013092107
Jia, B., Raphenya, A. R., Alcock, B., Waglechner, N., Guo, P., Tsang, K. K., Lago, B. A., Dave, B. M., Pereira, S., Sharma, A. N., Doshi, S., Courtot, M., Lo, R., Williams, L. E., Frye, J. G., Elsayegh, T., Sardar, D. Westman, E. L., Pawlowski, A. C., Johnson, T. A., Brinkman, F. S., Wright, G. D., and McArthur, A. G. (2017) CARD 2017: expansion and model-centric curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Research 45, D566-573. https://doi.org/10.1093/nar/gkw1004
Kocaoglu, O., and Carlson E.E. (2015) Profiling of β-Lactam Selectivity for Penicillin-Binding Proteins in Escherichia coli Strain DC2. Antimicrobial Agents and Chemotherapy 59, 2785-2790 https://doi.org/10.1128/aac.04552-14
LiverTox - Clinical and Research Information on Drug-Induced Liver Injury. National Institutes of Health. https://www.ncbi.nlm.nih.gov/books/NBK548463/
Piperacillin. Drugs.com https://www.drugs.com/cdi/piperacillin.html
Piperacillin - DrugBank. Drugbank.ca https://go.drugbank.com/drugs/DB00319
Piperacillin. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/43672
Sainsbury, S., Bird, L., Rao, V., Shepherd, S. M., Stuart, D. I., Hunter, W. N., Owens, R. J., Ren, J. (2011) Crystal structures of penicillin-binding protein 3 from Pseudomonas aeruginosa: comparison of native and antibiotic-bound forms. J Mol Biol. 405, 173-84. https://doi.org/10.1016/j.jmb.2010.10.024
Sutaria, D. S., Moya, B., Green, K. B., Kim, T. H., Tao, X., Jiao, Y., Louie, A., Drusano, G. L., Bulitta, J. B. (2018) First Penicillin-Binding Protein Occupancy Patterns of β-Lactams and β-Lactamase Inhibitors in Klebsiella pneumoniae. Antimicrob Agents Chemother. 62, e00282-18. https://doi.org/10.1128/aac.00282-18
van Berkel, S. S., Nettleship, J. E., Leung, I. K., Brem, J., Choi, H., Stuart, D. I., Claridge, T. D., McDonough, M. A., Owens, R. J., Ren, J., Schofield, C. J. (2013) Binding of (5S)-penicilloic acid to penicillin binding protein 3. ACS Chem Biol. 8, 2112-6. https://doi.org/10.1021/cb400200h
Zosyn (piperacillin and tazobactam for injection). (2017) Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050684s88s89s90_050750s37s38s39lbl.pdf
March 2025, Helen Gao, Shuchismita Dutta; Reviewed by Dr. Andrew Lovering
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/cellwall-biosynth/pbp/blm/piperacillin