Penicillin Binding Protein
Penicillin-binding proteins (PBPs) are enzymes found on the outer side of the cytoplasmic membrane, where they play an essential role in the last step of peptidoglycan biosynthesis. Because these enzymes are sensitive to penicillin and other β-lactam antibiotics, they are known as penicillin-binding proteins. PBPs are currently the target of US FDA approved β-lactam antibiotics. Some examples of these β-lactam antibacterial drugs include:
| Penicillin G (benzylpenicillin) | Methicillin | Ampicillin |
| Amoxicillin | Piperacillin | Cefoxitin |
| Ceftazidime | Cefepime | Aztreonam |
| Meropenem |
Overview of PBP Function
Peptidoglycan is a polymer consisting of alternating N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) glycan units that are crosslinked by peptide chains. The GlcNAc-MurNAc-pentapeptide unit, also known as lipid II, is formed by enzymes inside the cell. On the other hand, PBPs are generally responsible for two separate functions outside of the cytoplasmic membrane: catalyzing the polymerization of the glycan chains from the lipid II units and crosslinking two adjacent chains to form the characteristic mesh structure of peptidoglycan. It is important to note here that not all PBPs will carry out both of these functions. Some PBPs may be bifunctional and are responsible for both polymerization and crosslinking, whereas others may only catalyze crosslinking. There are also PBPs that are not directly involved in either of these processes but have entirely different functions, which will be discussed later on.
Transglycosylation
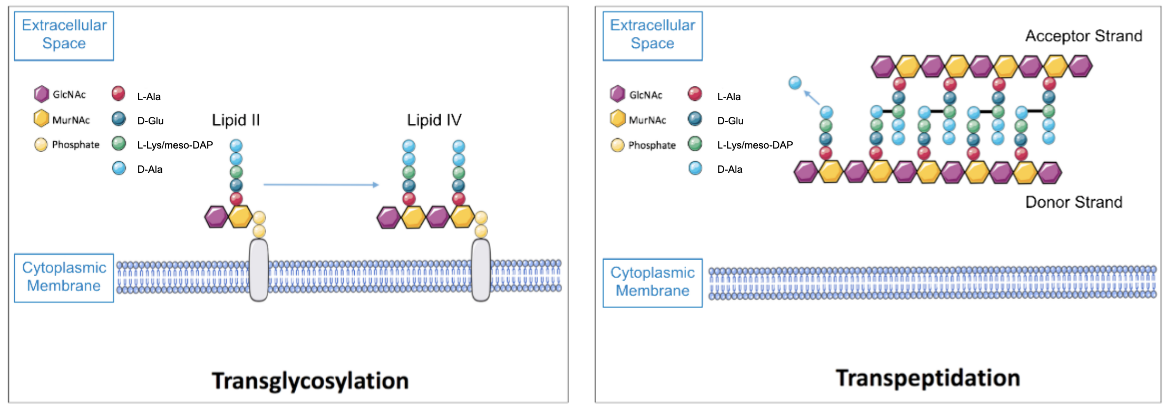
After lipid II, which consists of one GlcNAc and one MurNAc-pentapeptide unit bound together, is transferred outside of the cell into the extracellular region, the PBPs responsible for glycosyltransferase activity catalyze the polymerization of lipid II into a nascent peptidoglycan strand (Lovering et al., 2012). This process is initiated with the joining of two lipid II units to create a lipid IV product and continues with an elongation stage during which additional lipid II units are successively linked to form the glycan strand, as shown in Figure 1.
Transpeptidation
Once the glycan strands are produced by transglycosylation reactions, two adjacent strands are crosslinked by the PBPs responsible for transpeptidase activity. The crosslinking usually occurs between the fourth residue of the pentapeptide chain of the donor strand (D-Ala) and the third residue of the pentapeptide side chain of the acceptor strand (either L-Lys or meso-DAP, depending on whether the bacterium is gram positive or gram negative, respectively), as illustrated in Figure 1 (Lovering et al., 2012).
|
| Figure 1. A representation of the glycosyltransferase (left) and transpeptidase (right) reactions carried out by bifunctional PBPs. |
Classification of PBPs
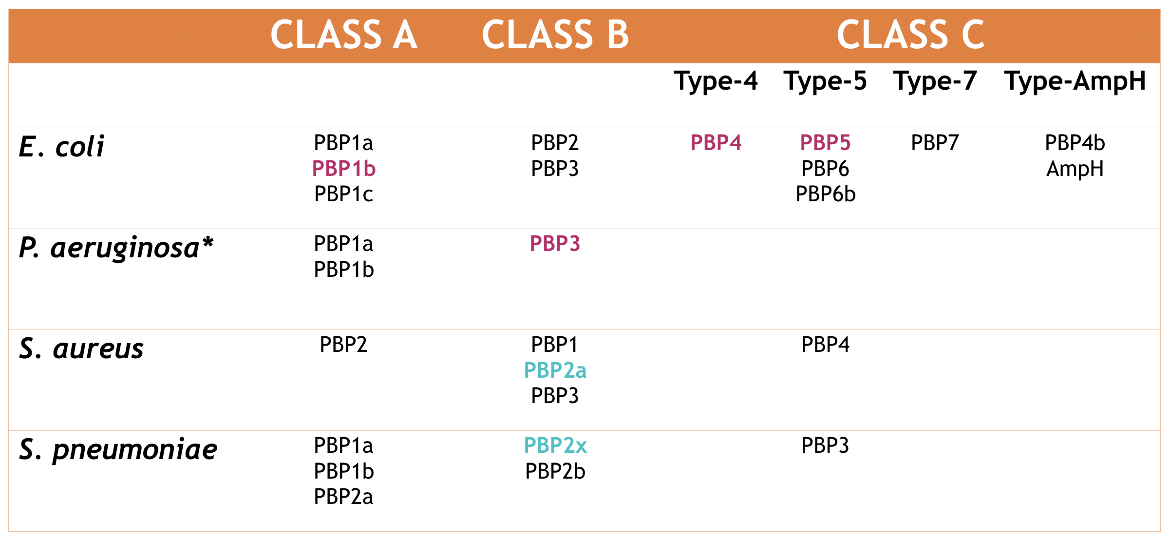
Different bacterial species possess varying numbers of PBPs. For instance, Escherichia coli (E. coli) bacteria have a total of 12 PBPs whereas Staphylococcus aureus (S. aureus) bacteria possess 5 PBPs at the most. Regardless of the number of PBPs bacteria possess, there is a classification system that applies to all the bacterial species that have these enzymes and that participate in peptidoglycan biosynthesis.
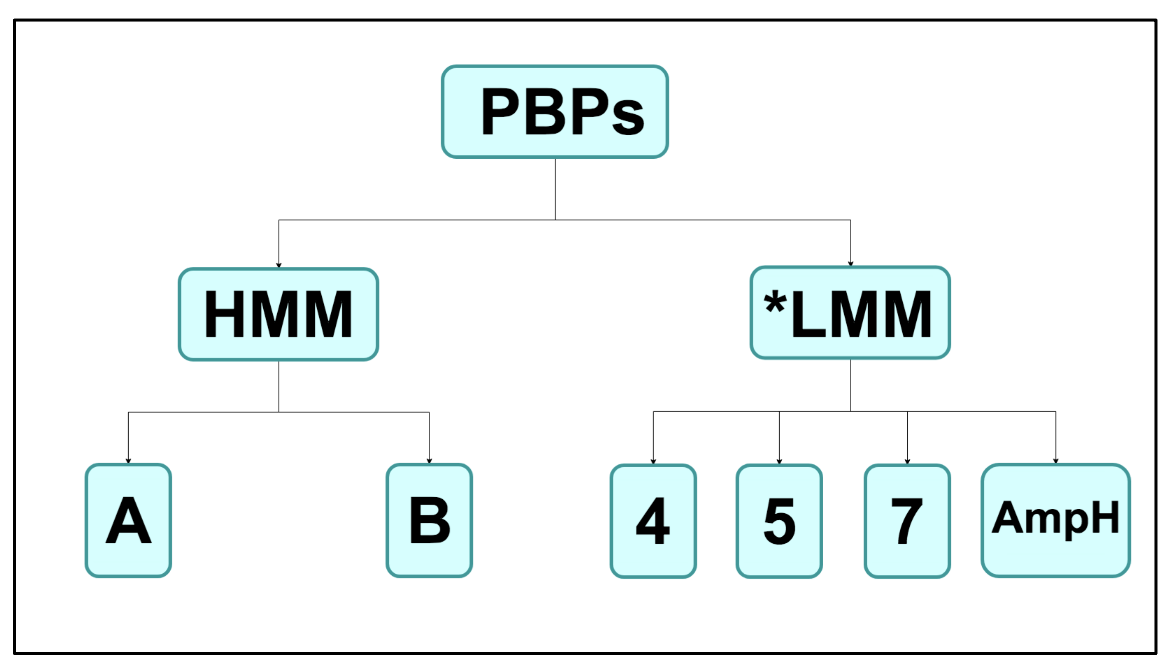
Primarily, PBPs are divided into two main groups: the high molecular mass (HMM) PBPs and the low molecular mass (LMM) PBPs (Sauvage et al., 2008). This division is not solely based on the molecular masses of the enzymes but rather a difference in the functional properties of the two groups as well. The HMM category is then further divided into class A and class B enzymes, and this distinction is based on the fact that class A PBPs can carry out both glycosyltransferase and transpeptidase activities whereas class B PBPs can catalyze only transpeptidation reactions (Sauvage et al., 2008). Unlike the HMM PBPs, LMM PBPs do not carry out transglycosylation or transpeptidation reactions. The function of LMM PBPs is less clear although it is known that they catalyze D,D-endopeptidase or D,D-carboxypeptidase activity to regulate the degree of crosslinking in peptidoglycan.
Due to the class A and class B distinction that occurs among HMM PBPs, the LMM PBPs are commonly known as class C PBPs as a whole (Sauvage et al., 2008). The class C enzymes of all bacterial species are then subdivided into type-4, type-5, type-7, and type-AmpH PBPs based on their similarity to the E. coli PBP4, PBP5, PBP7, and AmpH enzymes, respectively (Sauvage et al., 2008).
|
| Figure 2. A chart depicting the classification of the PBPs. *LMM PBPs are commonly known as class C PBPs as well. |
The classification of all the PBPs found in four different bacterial organisms can be seen in Figure 3. The numbering system for PBPs is based historically on an electrophoresis method. Therefore, although E. coli class A PBP1a is functionally similar to S. aureus class A PBP2 (Sauvage et al., 2008), the numbering for both enzymes is not the same.
Class A PBPs
Structure
Class A PBPs catalyze both transglycosylation and transpeptidation reactions and thus possess two distinct domains that each carry out one of these functions. The N-terminal domain is responsible for glycosyltransferase activity and polymerization of glycan chains (Sauvage et al., 2008). The C-terminal domain is known as the transpeptidase domain due to its ability to catalyze the crosslinking of adjacent glycan strands. It is also referred to as the penicillin-binding domain since penicillin and other β-lactam antibiotics can bind to the active site of this domain and prevent the enzyme from catalyzing transpeptidation.
E. coli bacteria produce three class A PBPs: PBP1a, PBP1b, and PBP1c. PBP1a and PBP1b are the major enzymes that catalyze transglycosylation and transpeptidation in the bacterial cell (Sauvage et al., 2008). Bacterial cells will be viable as long as either PBP1a or PBP1b is present, which demonstrates the overlapping function of both proteins (King et al., 2016). The role of PBP1c is less clearly understood (Sauvage et al., 2008).
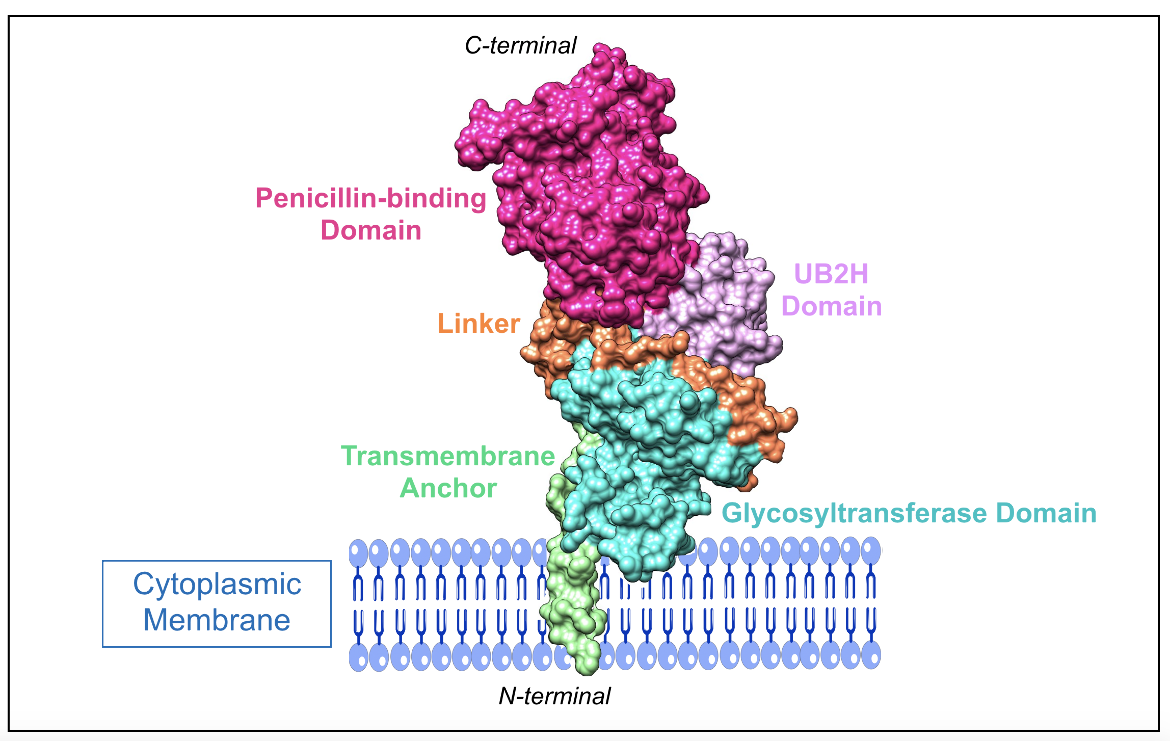
E. coli PBP1b is a bifunctional class A enzyme, and it is made up of five domains (King et al., 2016), which are:
1. N-terminal transmembrane anchor (residues 64-87)
2. UvrB domain 2 homolog (UB2H) domain (residues 109-200)
3. Membrane-associated N-terminal glycosyltransferase domain (residues 203-367)
4. Linker region connecting the glycosyltransferase and penicillinbinding domains (residues 391-443)
5. C-terminal penicillin-binding domain (residues 444-736)
In addition to the penicillin-binding domain and glycosyltransferase domain, PBP1b has an additional unique domain known as UB2H. It is thought that it interacts with membrane-bound regulatory proteins (King et al., 2016), which activates the enzyme and stimulates peptidoglycan synthesis (Egan et al., 2014).
Function
Active Site of the Glycosyltransferase Domain
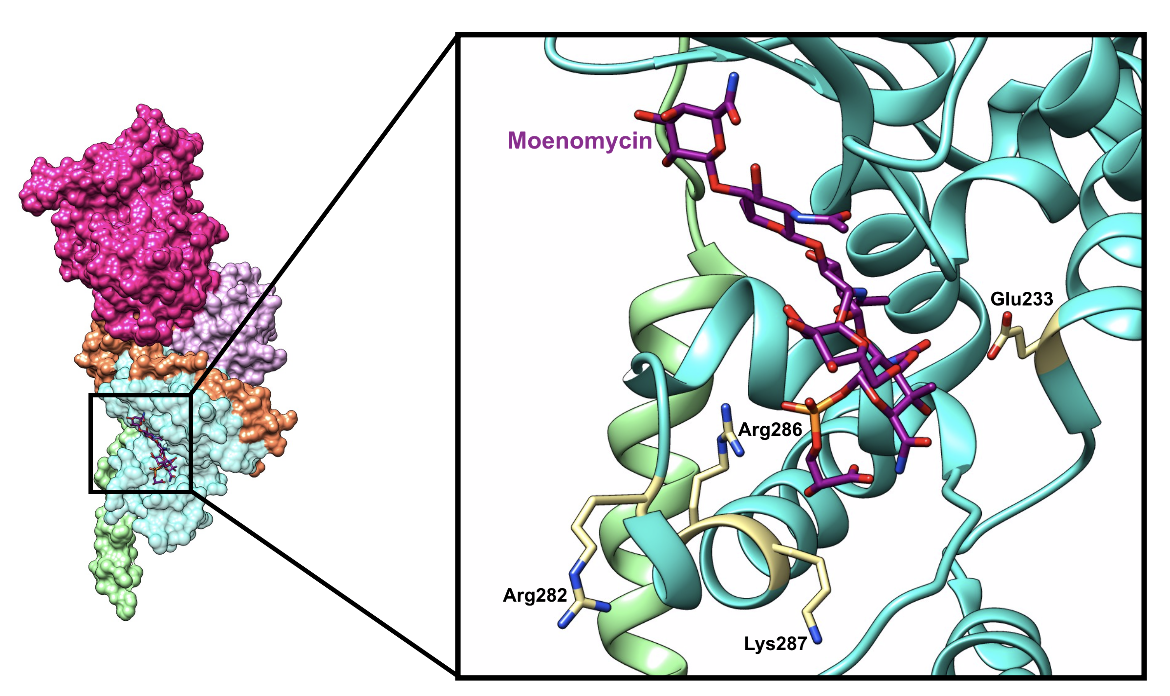
This domain catalyzes the glycosidic bond formation between two lipid II units as follows. First, a lipid II unit binds to the donor site and a second lipid II unit binds at the acceptor site of the domain. A Glu233 residue is positioned to deprotonate the C4 hydroxyl group of the GlcNAc found in the lipid II unit bound to the acceptor site. This action produces a nucleophile that attacks the C1 MurNAc group of the lipid II unit in the donor site (Terrak et al., 2008). As a result, a covalent glycosidic bond is formed and the donor lipid II loses its C55-PP group (King et al., 2016). The product at the acceptor site is moved to the donor site, which is facilitated by Arg286, Lys287, and Arg282 in the case of PBP1b, as shown in Figure 6a. Each reaction produces free undecaprenyl pyrophosphate, which is "flipped" back toward the cytoplasmic side of the inner membrane, where it is hydrolyzed to undecaprenyl phosphate and recycled in a new reaction (Macheboeuf et al., 2006).
An inhibitor of the glycosyltransferase domain is moenomycin, which binds to the donor active site by mimicking the natural lipid II substrate, as illustrated below in Figure 5.
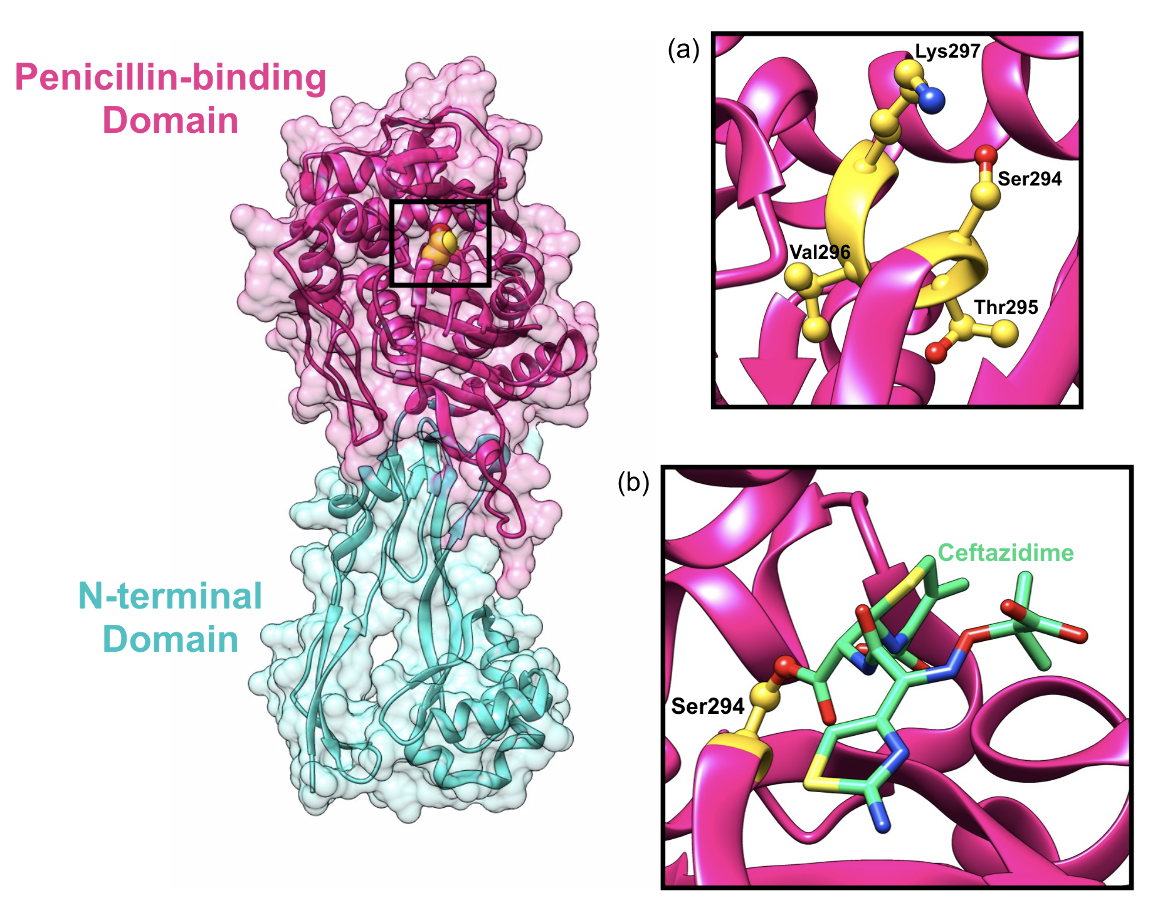
Active Site of the Penicillin-binding (Transpeptidase) Domain
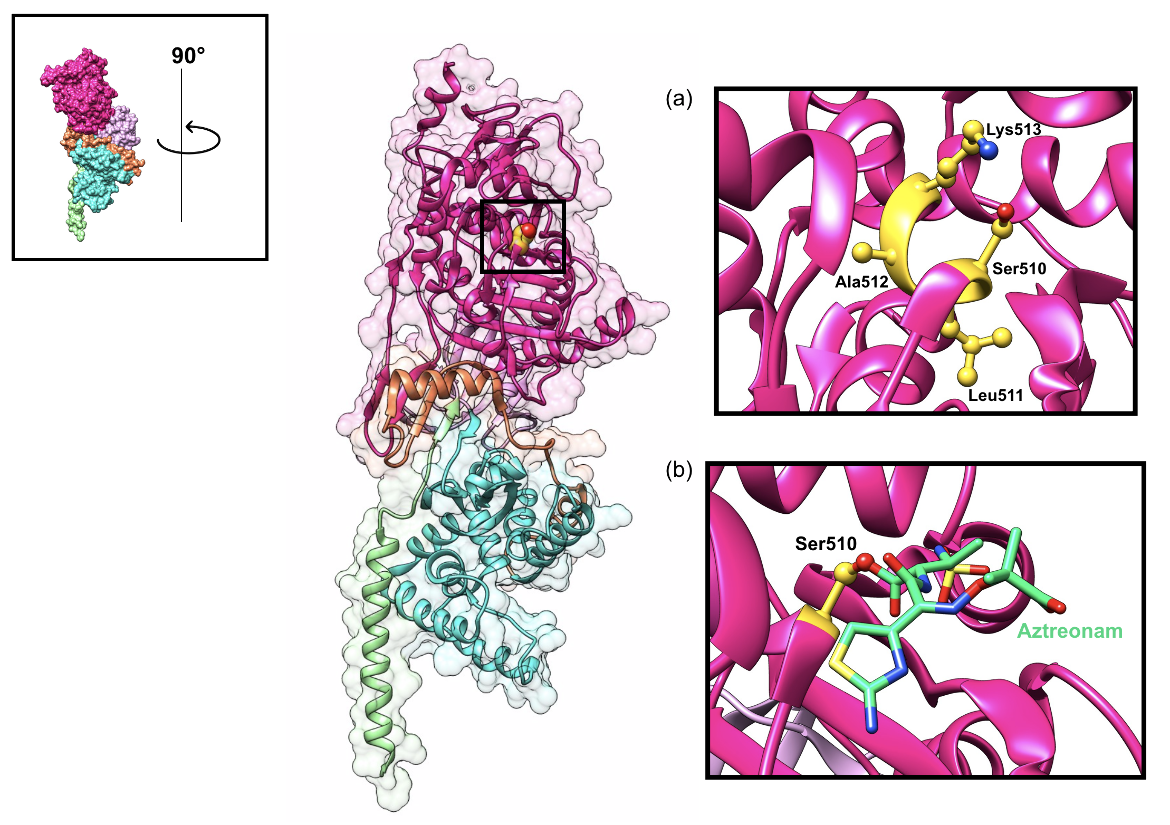
Transpeptidation relies on a few key residues found in three conserved motifs (SxxK, SxN, and KT/GT) that constitute the active site. It is the serine residue of the SxxK motif that plays a crucial role in the two-step crosslinking reaction (King et al., 2016). The catalytic serine residue binds to the C-terminal D-alanine D-alanine peptide bond of the donor nascent glycan strand. The terminal D-alanine residue is released. The resulting tetrapeptide forms an acyl-enzyme intermediate that can now be crosslinked with the acceptor glycan strand. β-lactam antibiotics, such as aztreonam, mimic the shape of the terminal D-alanine D-alanine peptide bond and bind to the catalytic serine residue, which prevents the enzyme from interacting with its natural substrate and catalyzing transpeptidation, as shown in Figure 6b.
Pharmacological Implications
Unlike class B and class C enzymes, class A PBPs have an additional catalytic domain that can be a potential target of novel antibacterial drugs: the glycosyltransferase domain. Currently, it is known that moenomycin is an analogue of the lipid II substrate that binds to the active site of the glycosyltransferase domain and inhibits transglycosylation. Inhibition by moenomycin compromises the second to last step in peptidoglycan biosynthesis, resulting in bacterial cell lysis and death. Moenomycin is not used clinically due to its poor pharmacokinetic properties; however, it serves as a blueprint for future antibacterial agents.
See Pharmacological Implications of Class B PBPs to read about the implications of inhibiting the transpeptidase domain.
Class B PBPs
Structure
Like class A PBPs, class B enzymes also have a C-terminal penicillin-binding domain and an N-terminal domain. However, Class B PBPs are monofunctional transpeptidases since their N-terminal domain has no enzymatic activity and does not catalyze transglycosylation (Sauvage et al., 2008). The N-terminal domain of class B PBPs is thought to serve as a stalk, which allows the C-terminal penicillin-binding domain to reach the peptidoglycan (King et al., 2016). In addition, this domain has been suggested to play a role in cell morphogenesis by potentially interacting with other proteins involved in the bacterial cell cycle (Sauvage et al., 2008). In PBP2a, it presents a binding site for ceftaroline that plays an important allosteric role in inhibiting the active site ~60Å away (Fishovitz et al., 2014).
PBP2X from S. pneumoniae and PBP2a from S. aureus are two examples of monofunctional class B PBPs that catalyze transpeptidation. It is important to note that mutations in PBP2X confer increased resistance levels to S. pneumoniae strains without changes in other PBP or non-PBP genes (Mousavi et al., 2017). Similarly, the key determinant of broad-spectrum β-lactam resistance seen in methicillin-resistant S. aureus (MRSA) strains is PBP2a (Lim and Strynadka, 2002). The enzyme has a low affinity for β-lactams; therefore, it is still able to catalyze transpeptidation even in the presence of β-lactam antibiotics (Lim and Strynadka, 2002).
Function
Active Site of the Penicillin-binding (Transpeptidase) Domain
Similar to the penicillin-binding domain of class A PBP enzymes, the penicillin-binding domain of class B PBPs are responsible for catalyzing the transpeptidation reaction, which is dependent on the same three conserved motifs (SxxK, SxN, and KT/GT). The first D-alanine residue of the donor strand enters the active site of the PBP and forms a noncovalent, reversible complex (Sauvage et al., 2008). The nucleophilic serine residue attacks the carbonyl group carbon of the penultimate D-alanine residue, cleaving the terminal D-alanine of the pentapeptide side chain. The third residue in the pentapeptide chain of a donor glycan strand donates a proton, which allows for the release of the donor strand and crosslinking between the fourth residue of the donor strand and the third residue of the acceptor strand (Sauvage et al., 2008). Similar to other β-lactam antibiotics, ceftazidime mimics the D-alanine D-alanine terminal end of the peptide side chain and binds to the catalytic serine residue (Figure 7b), which inhibits the transpeptidase domain.
One example of a class B PBP is PBP3 from P. aeruginosa which is also found anchored to the cytoplasmic membrane similar to class A PBPs and consists of two domains (Han et al., 2010):
1. N-terminal nonpenicillin-binding domain (residues 1-224)
2. C-terminal penicillin-binding domain (residues 225-579)
Pharmacological Implications
The transpeptidase domain of PBPs is the current target of US FDA approved β-lactam antibiotics. It is advantageous to design drugs that target these specific enzymes because they are already found outside of the cytoplasmic membrane. This allows the antibacterial agents to access their targets more easily (Sauvage et al., 2016). β-lactams block the enzyme in an inactive form through acylation of the catalytic serine, which inhibits transpeptidase activity (Sauvage et al., 2016). By preventing transpeptidation, the robust mesh structure of peptidoglycan cannot be formed, which in turn compromises the integrity of the cell wall and results in bacterial death.
Class C PBPs
Structure and Function
As was previously noted, class C PBPs catalyze neither transglycosylation nor transpeptidation. As a matter of fact, it has been concluded that all seven class C PBPs of E. coli are nonessential to the bacteria. This means that bacterial cells missing all seven genes for these PBPs are able to survive and grow in a rich medium (Kishida et al., 2006). As long as either class A PBP1a or PBP1b is present along with class B PBP2 and PBP3 in E. coli, the bacteria will be able to carry out the glycosyltransferase and transpeptidase activities required to synthesize peptidoglycan (Kishida et al., 2006).
Type-4 PBPs
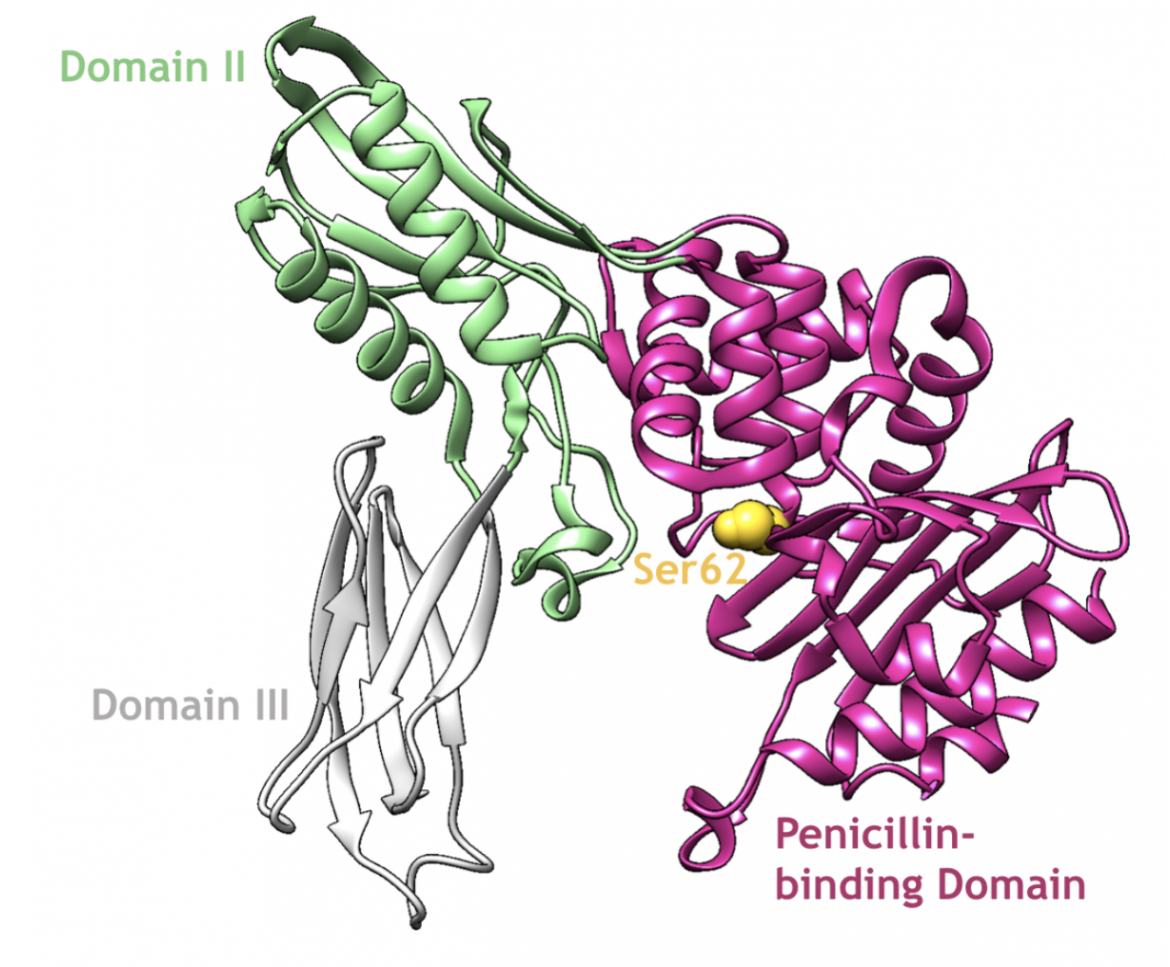
In general, there are either one or zero type-4 PBPs found in bacteria. One such example of a type-4 class C PBP is PBP4 from E. coli, which is made up of three distinct domains (Kishida et al., 2006):
1. Penicillin-binding domain (residues 1-80, 294-477)
2. Domain II (residues 81-172, 248-293)
3. Domain III (residues 173-247)
|
| Figure 8. A ribbon representation of PBP4, which is a type-4 class C enzyme from E. coli, and its catalytic serine residue (PDB ID: 2ex2; Kishida et al., 2006). |
Type-4 class C PBPs are not anchored to the cytoplasmic membrane like class A and class B enzymes. This means that these PBPs could be inserted into the peptidoglycan or exposed on the outer face of the peptidoglycan, where they could catalyze endopeptidase activity (Sauvage et al., 2008). Endopeptidation occurs when type-4 PBPs cleave crosslinked side chains that were previously linked by one or more of the HMM PBPs during peptidoglycan synthesis (Meberg et al., 2004) so that new glycan chains can be inserted (Kishida et al., 2006).
In addition to D,D-endopeptidase activity, type-4 class C PBPs may also carry out D,D-carboxypeptidation, which removes the terminal D-alanine residue from the short peptide side chain that extends from each MurNAc moiety (Meberg et al., 2004). Similar to transpeptidation, class C PBP enzymes need to recognize the terminal D-alanine, D-alanine moiety of the pentapeptide chain and catalyze a nucleophilic attack of the carbonyl group of the penultimate D-alanine, which is performed by the active serine residue (Macheboeuf et al., 2006). The difference in a carboxypeptidation reaction is that the acceptor in the reaction is a water molecule (Macheboeuf et al., 2006). As a result, the pentapeptide chain is hydrolyzed into a tetrapeptide after D-alanine is released, and further peptidoglycan crosslinking is prevented (Macheboeuf et al., 2006). By hydrolyzing the peptide stem of peptidoglycan, endopeptidases and carboxypeptidases act as autolysins, which are responsible for cell wall remodeling. Mutant strains lacking one or more PBPs are able to grow normally; however, those lacking multiple autolysins are seen to exhibit abnormal morphologies (Johnson et al., 2012).
Type-5 PBPs
PBP5 and PBP6 are the primary class C PBP enzymes strictly responsible for D,D-carboxypeptidase activity in E. coli. The structure of PBP5 is composed of two domains:
1. Penicillin-binding domain (residues 3-262)
2. C-terminal domain (residues 263-356)
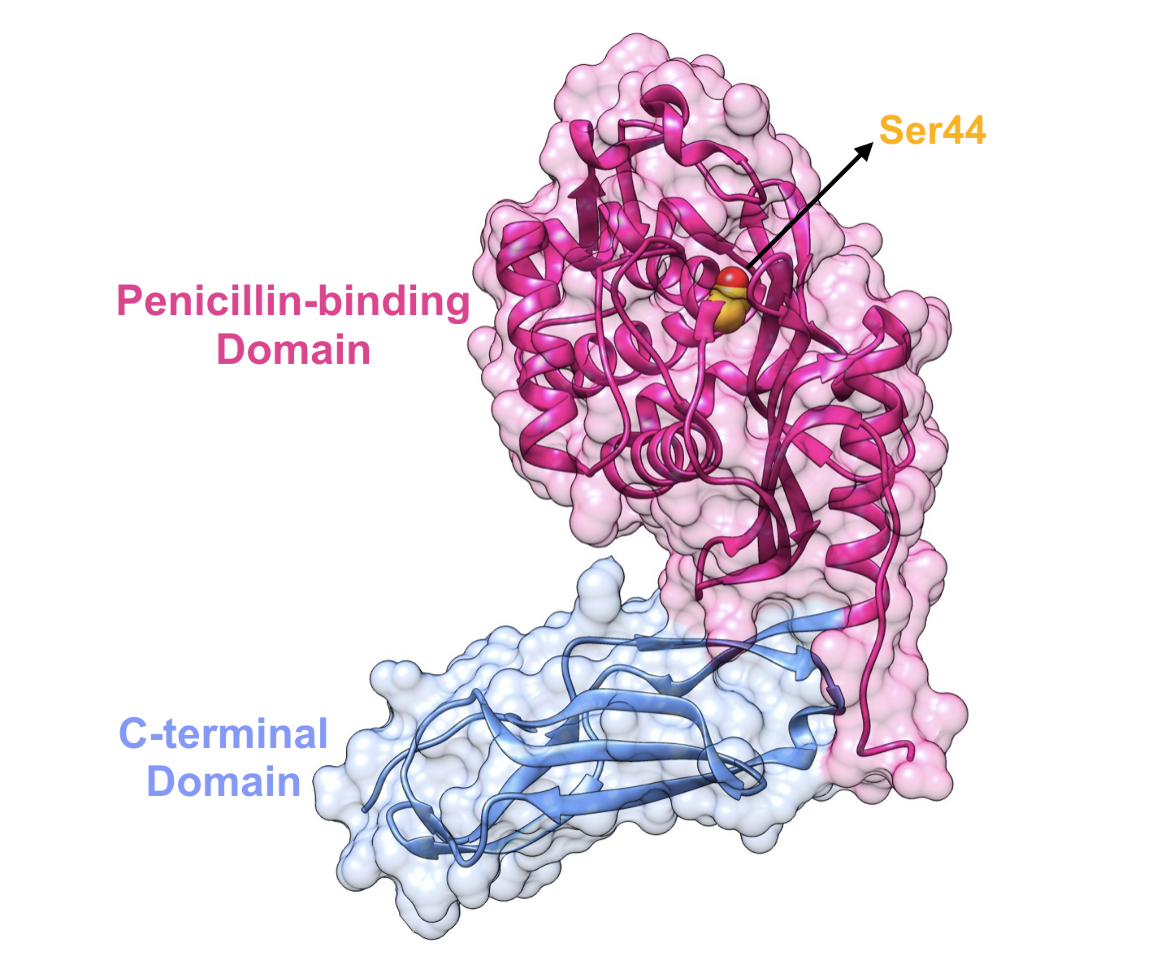
|
| Figure 9. Representation of the two-domain structure of E. coli PBP5. The nucleophilic serine residue in the active site is highlighted in yellow (PDB ID: 3mze; Nicola et al., 2010). |
PBP5 is the major carboxypeptidase in E. coli, and although it is the most abundant of the PBPs, its loss is not lethal to the bacterium (Nelson and Young, 2000). However, mutants lacking PBP5 do exhibit morphological abnormalities, such as altered diameters and topological features (Nelson and Young, 2000). This suggests that PBP5 plays an important role in the maintenance of bacterial cell shape (Nelson and Young, 2000).
Pharmacological Implications
Inhibiting either transpeptidation or carboxypeptidation will weaken the peptidoglycan layer, thus compromising the integrity of the cell wall. β-lactams that bind to the penicillin-binding domain of class C PBPs prevent the remodeling and recycling of the peptidoglycan layer, which is not necessarily lethal on its own. However, combined with the antibiotic molecules that bind to the penicillin-binding domain of class A and class B PBPs, the antibiotics that bind to class C PBPs, will have a bactericidal effect.
References
Egan, A. J., Jean, N. L., Koumoutsi, A., Bougault, C. M., Biboy, J., Sassine, J., Solovyova, A. S., Breukink, E., Typas, A., Vollmer, W., Simorre, J. P. (2014). Outer-membrane lipoprotein LpoB spans the periplasm to stimulate the peptidoglycan synthase PBP1B. Proceedings of the National Academy of Sciences of the United States of America, 111(22), 8197–8202. https://doi.org/10.1073/pnas.1400376111
Fishovitz, J., Rojas-Altuve, A., Otero, L. H., Dawley, M., Carrasco-López, C., Chang, M., Hermoso, J. A., Mobashery, S. (2014) Disruption of allosteric response as an unprecedented mechanism of resistance to antibiotics. J Am Chem Soc. 136(28):9814-7. https://doi.org/10.1021/ja5030657
Han, S., Zaniewski, R. P., Marr, E. S., Lacey, B. M., Tomaras, A. P., Evdokimov, A., Miller, J. R., Shanmugasundaram, V. (2010) Structural basis for effectiveness of siderophore-conjugated monocarbams against clinically relevant strains of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A., 107, 22002-7. https://doi.org/10.1073/pnas.1013092107
Johnson, J. W., Fisher, J. F., Mobashery, S. (2012). Bacterial cell-wall recycling. Annals of the New York Academy of Sciences, 1277(1), 54–75. https://doi.org/10.1111/j.1749-6632.2012.06813.x
King, D. T., Wasney, G. A., Nosella, M., Fong, A., Strynadka, N. C. (2016). Structural Insights into Inhibition of Escherichia coli Penicillin-binding Protein 1B. The Journal of biological chemistry, 292(3), 979–993. https://doi.org/10.1074/jbc.M116.718403. PDB ID: 5hlb
Kishida, H., Unzai, S., Roper, D. I., Lloyd, A., Park, S. Y., Tame, J. R., (2006) Crystal structure of penicillin binding protein 4 (dacB) from Escherichia coli, both in the native form and covalently linked to various antibiotics. Biochemistry 45: 783– 792. https://doi.org/10.1021/bi051533t PDB ID: 2ex2
Lim, D., Strynadka, N. C. (2002). Structural basis for the beta lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nature structural biology, 9(11), 870–876. https://doi.org/10.1038/nsb858
Lovering, A. L., Safadi, S. S., Strynadka, N. C. (2012). Structural perspective of peptidoglycan biosynthesis and assembly. Annual review of biochemistry, 81, 451-478. https://doi.org/10.1146/annurev-biochem-061809-112742
Macheboeuf, P., Contreras-Martel, C., Job, V., Dideberg, O., Dessen, A. (2006). Penicillin binding proteins: key players in bacterial cell cycle and drug resistance processes. FEMS microbiology reviews, 30(5), 673–691. https://doi.org/10.1111/j.1574-6976.2006.00024.x
Mousavi, S. F., Pana, M., Feizabadi, M., Jalali, P., Ghita, M., Denapaite, D., Hakenbeck, R. (2017). Diversity of Mosaic pbp2x Families in Penicillin-Resistant Streptococcus pneumoniae from Iran and Romania. Antimicrobial agents and chemotherapy, 61(12), e01535-17. https://doi.org/10.1128/AAC.01535-17
Nelson, D. E., Young, K. D. (2000). Penicillin binding protein 5 affects cell diameter, contour, and morphology of Escherichia coli. Journal of Bacteriology, 182(6), 1714-1721. https://doi.org/10.1128/jb.182.6.1714-1721.2000
Nicola, G., Tomberg, J., Pratt, R. F., Nicholas, R. A., Davies, C. (2010). Crystal structures of covalent complexes of β-lactam antibiotics with Escherichia coli penicillin-binding protein 5: toward an understanding of antibiotic specificity. Biochemistry, 49(37), 8094-8104. https://doi.org/10.1021/bi100879m
Sainsbury, S., Bird, L., Rao, V., Shepherd, S. M., Stuart, D. I., Hunter, W. N., Owens, R. J., Ren, J. (2011) Crystal structures of penicillin-binding protein 3 from Pseudomonas aeruginosa: comparison of native and antibiotic-bound forms. J Mol Biol. 405, 173-84. https://doi.org/10.1016/j.jmb.2010.10.024
Sauvage, E., Kerff, F., Terrak, M., Ayala, J. A., Charlier, P. (2008). The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS microbiology reviews, 32(2), 234-258. https://doi.org/10.1111/j.1574-6976.2008.00105.x
Terrak, M., Sauvage, E., Derouaux, A., Dehareng, D., Bouhss, A., Breukink, E., et al. (2008). Importance of the conserved residues in the peptidoglycan glycosyltransferase module of the class A penicillin-binding protein 1b of Escherichia coli. J. Biol. Chem. 283, 28464–28470. https://doi.org/10.1074/jbc.m803223200
March 2025, Gauri Patel, Helen Gao; Reviewed by Dr. Andrew Lovering
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/cellwall-biosynth/pbp