Ceftazidime
Drug Name
Ceftazidime is a semi-synthetic bactericidal antibiotic, which is classified as a third-generation cephalosporin. It has broad-spectrum activity against a wide range of gram-negative bacteria in vitro and has been shown to be active against gram-positive bacteria as well (DrugBank). However, because ceftazidime has weaker activity against gram-positive pathogens, it is typically not used to treat such infections (CARD, 2017). Ceftazidime is also one of the few cephalosporin antibiotics that is active against Pseudomonas aeruginosa infections (Kos et al., 2016).
Table 1. Basic profile of ceftazidime.
| Description | Intravenously (IV) or intramuscularly (IM) administered, semi-synthetic broad-spectrum antibacterial drug |
| Target(s) | Penicillin-binding proteins (PBPs); primarily penicillin-binding protein 3 (PBP3) |
| Generic | Ceftazidime for injection |
| Commercial Name | Fortaz® (United States, Canada) |
| Combination Drug(s) | Avycaz® (ceftazidime & avibactam; United States) |
| Other Synonyms | CAZ, Ceftazidim, Ceftazidima, Ceftazidime anhydrous, Ceftazidimum |
| IUPAC Name | 1-{[(6R,7R)-7-[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(1-carboxy-1-methylethoxy)imino]acetamido]-2-carboxylato-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl}pyridin-1-ium |
| Ligand Code in PDB | CTJ (bound form), CAZ (Acylated form) |
| 3D Structure | 3ocn (ceftazidime bound to target protein PBP3) |
| ATC code | J01DD02 |
|
|
|
Antibiotic Chemistry
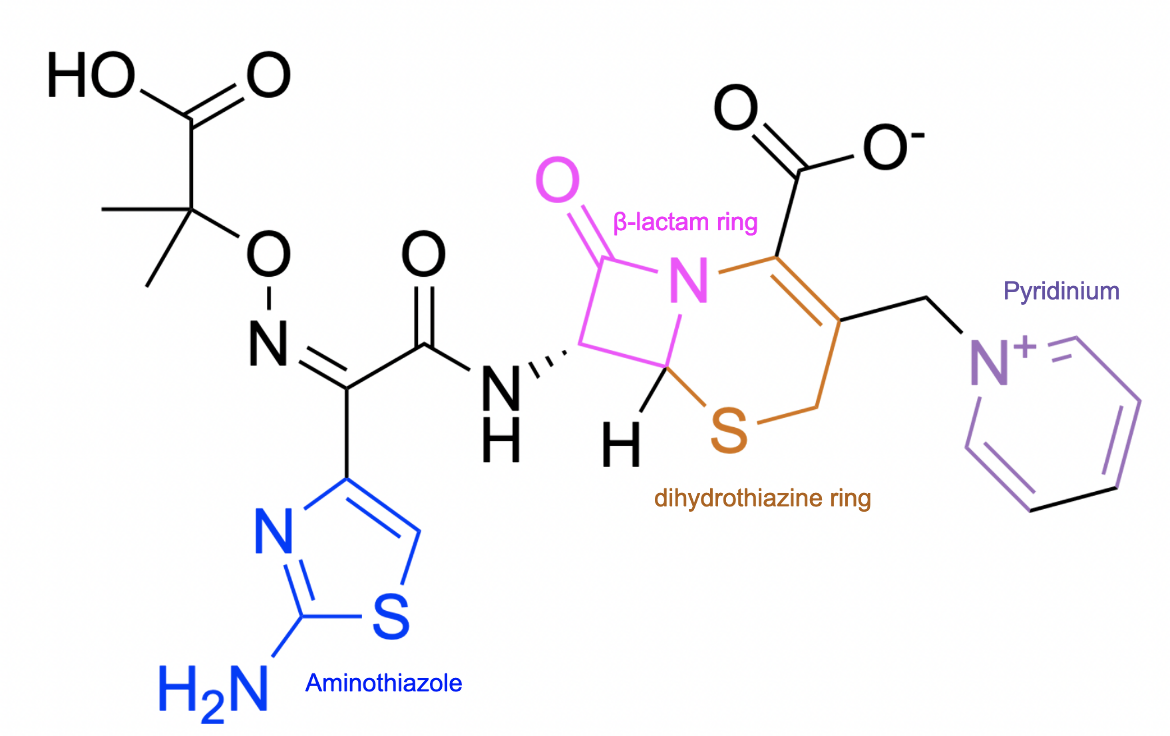
Ceftazidime is composed of a β-lactam ring (shown in pink in Figure 2) fused to a six-membered dihydrothiazine ring (shown in orange in Figure 2), which is a characteristic of all cephalosporin antibiotics. Certain antibacterial properties of ceftazidime can be attributed to a few of its additional structural features. For instance, the charged pyridinium ring increases the antibiotic's solubility, and the aminothiazole group increases the stability of the molecule.
|
| Figure 2. 2D structure of ceftazidime highlighting functional moieties responsible for antibacterial activity. Structure created using ChemDraw. |
Drug Information
Table 2. Chemical and physical properties (DrugBank).
| Chemical Formula | C22H22N6O7S2 |
| Molecular Weight | 546.6 g/mol |
| Calculated Predicted Partition Coefficient: cLogP | -1.2 |
| Calculated Predicted Aqueous Solubility: cLogS | -5 |
| Solubility (in water) | 0.00573 mg/mL |
| Predicted Topological Polar Surface Area (TPSA) | 191.22 Å2 |
Drug Target
Ceftazidime disrupts cell wall biosynthesis in bacteria by inhibiting enzymes known as penicillin-binding proteins (PBPs). This name originates from the ability of penicillin and other β-lactam antibiotics to bind to these proteins. Some of these PBP enzymes are responsible for catalyzing the final steps of the peptidoglycan synthesis pathway, which include polymerizing glycan strands and then cross-linking adjacent chains to form the characteristic mesh structure of peptidoglycan. On the other hand, other PBPs are involved in regulating peptidoglycan recycling and cell wall remodeling. Peptidoglycan, which is a polymer consisting of amino acids (peptido-) and sugars (-glycan), is the major component of the bacterial cell wall, and its mesh-like structure provides the cell wall with structure and rigidity. However, inhibition of those PBPs involved only in peptidoglycan remodeling is non-lethal to the bacteria.
Learn more about PBPs.
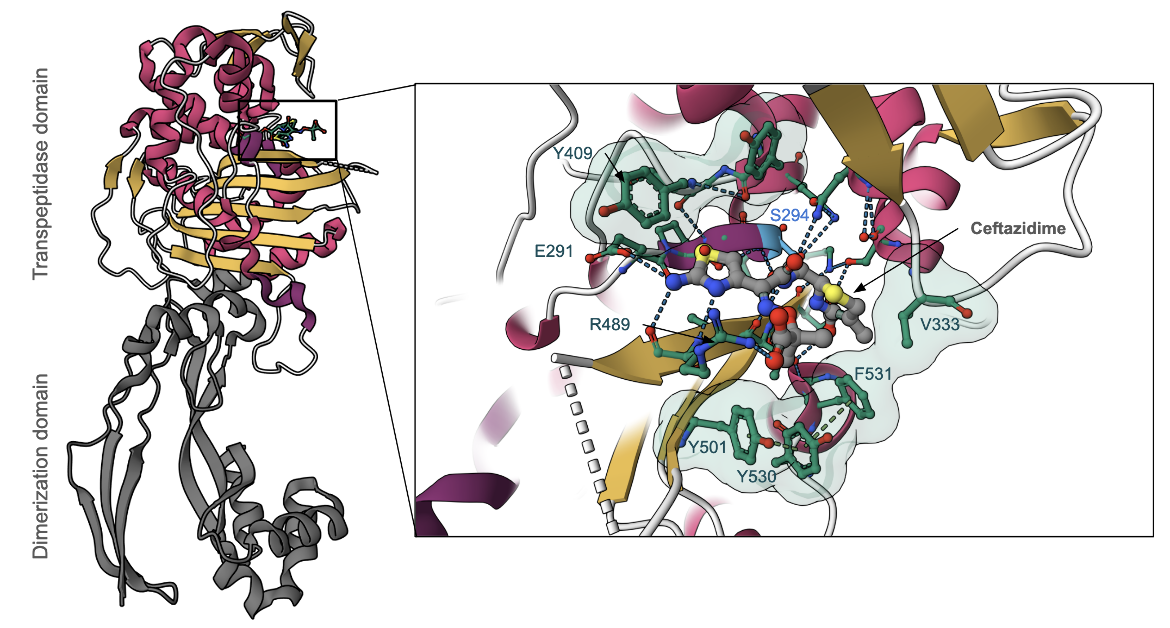
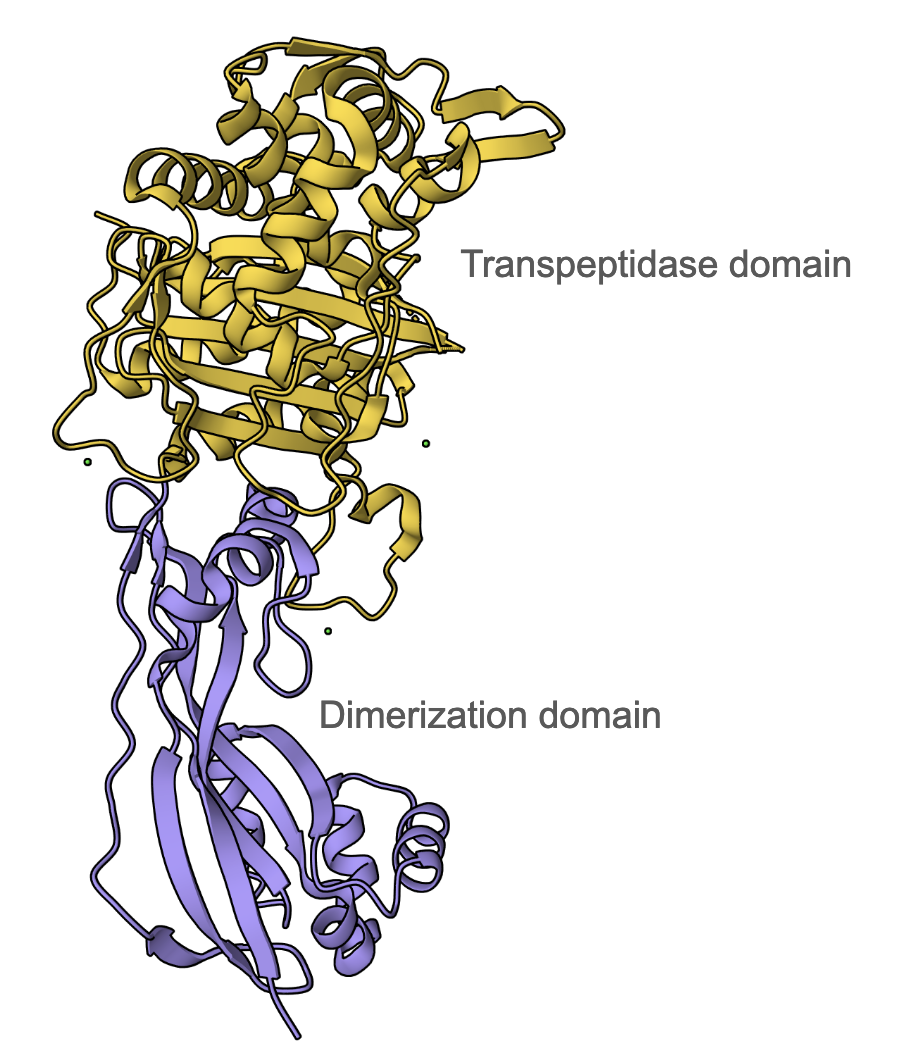
Ceftazidime primarily binds to PBP3 (DrugBank); therefore, the discussion here will be focused on how the antibiotic inhibits this enzyme in P. aeruginosa. The PBP3 protein has two main domains - an N-terminal dimerization domain (residues 35-207, colored mauve in Figure 3) and a transpeptidase domain (residues 232-532, colored yellow in Figure 3). PBP3 serves as a transpeptidase during peptidoglycan synthesis. It is able to catalyze cross-linking between peptide substituents of the glycan strands to form the mesh-like structure of peptidoglycan. It is a monofunctional enzyme because it is only involved in cross-linking, or transpeptidation, not in polymerizing the nascent glycan strands, a process known as transglycosylation. The Ser294 nucleophile is where the D-Ala-D-Ala peptide bond of the glycan strand binds in the active site and takes part in the transpeptidation reaction.
Inhibition of the transpeptidase function of this PBP3 (which is responsible for cross-linking), results in a severely weakened cell wall, which then causes bacterial cell lysis and death (PFam).
|
| Figure 3: Overall structure of P. aeruginosa PBP3 (PDB ID 3oc2, Sainsbury et al., 2011), showing 2 domains - mauve: dimerization domain and yellow: transpeptidase domain. |
Drug-Target Complex
The penicillin-binding domain in PBP3 of P. aeruginosa is where β-lactam antibiotics, such as ceftazidime, bind (Figure 4). Ceftazidime is able to inhibit PBP3 because its β-lactam ring is a structural mimic of the backbone of the D-Ala-D-Ala peptide bond, which allows the antibiotic to occupy the same binding site as the natural substrate. Specifically, the antibiotic reacts with the Ser294 nucleophile and forms an acyl-enzyme covalent complex (Figure 3). The natural substrate can no longer access the active site of PBP3 since the β-lactam blocks the Ser294 residue, which thus inactivates the enzyme and prevents transpeptidation.
The active site consists of a few important amino acid side chains that surround the bound antibiotic (Figure 4, Sainsbury et al., 2011). Primarily, when ceftazidime binds, the Tyr409 side chain, which is originally blocking the aminothiazole binding site in the apo-enzyme is swung outward to avoid a steric clash (Figure 4 inset). This Tyr409 residue also stabilizes the aminothiazole moiety through a hydrophobic interaction. There are also additional hydrogen bond interactions that ceftazidime makes with amino acid side chains (e.g., Glu291 and Arg489) in the binding pocket.
Moreover, Tyr530 and Phe531 also adopt a different conformation after ceftazidime covalently binds, which causes the Tyr501 side chain to move toward the active site. Together, these three side chains interact via pi-stacking and produce a unique hydrophobic aromatic wall around the gem-dimethyl group. The aromatic wall is further stabilized by Val333 which shifts away from the active site upon binding. In addition, Arg489 has a hydrogen bonding interaction with the carboxylic acid group of the gem-dimethyl moiety. The aromatic wall, along with interactions with Val333 and Arg489, all contribute to the efficient inhibition of PBP3 by ceftazidime (Figure 4, Sainsbury et al., 2011).
Pharmacologic Properties and Safety
Warning: Careful inquiry should be made to determine whether a patient has had previous hypersensitivity reactions to other cephalosporin or penicillin antibiotics prior to administering ceftazidime.
Table 3. Pharmacokinetics: ADMET of ceftazidime.
| Features | Comment(s) | Source |
|---|---|---|
| Absorption | Absorption is directly proportional to the size of the dose. | DrugBank |
| IC50 (µg/mL) | 0.1 µg/mL (for binding to PBP3 of P. aeruginosa) | (Davies et al., 2007) |
| Ki (µM) | N/A | N/A |
| Half-life (hrs) | ~1.9 hours (following IV administration) ~2 hours (following IM administration) | FDA |
| Duration of Action | 8-12 hours | FDA |
| Absorption Site | N/A | N/A |
| Transporter(s) | N/A | N/A |
| Metabolism | Not metabolized | Drugs.com |
| Excretion | ~ 80% to 90% of an IV or IM dose is excreted unchanged by the kidneys over a 24-hour period; Elimination of ceftazidime by the kidneys resulted in high therapeutic concentrations of the drug in urine; Ceftazidime is excreted in human milk in low concentrations. | FDA |
| AMES Test (Carcinogenic Effect) | Probability 0.7979 (Non AMES toxic) | DrugBank |
| hERG Safety Test (Cardiac Effect) | Probability 0.9938 (weak inhibitor) | DrugBank |
| Liver Toxicity | In general, cephalosporins are associated with little hepatotoxicity. Cases of cephalosporin-induced liver injury have rarely been reported. | LiverTox |
Parenteral administration of cephalosporins can result in minor elevations in serum aminotransferase and alkaline phosphatase values. However, these increases are usually mild and asymptomatic and do not cause more severe liver injury (LiverTox). In addition, the elevations generally resolve after ceftazidime therapy is discontinued (Drugs.com).
The serum half-life of ceftazidime is significantly prolonged in patients with renal impairment since the drug is eliminated almost exclusively by the kidneys (FDA, 2017). On the other hand, the pharmacokinetics of ceftazidime were unchanged in patients with hepatic dysfunction (FDA, 2017). Therefore, as long as renal function is not impaired as well, a dosage adjustment is not typically required for patients with hepatic dysfunction (FDA, 2017).
Drug Interactions and Side Effects
Table 4. Drug interactions and side effects of ceftazidime.
| Features | Comment(s) | Source |
|---|---|---|
| Total Number of Drug Interactions | 27 drugs | Drugs.com |
| Major Drug Interaction(s) | bcg; cholera vaccine, live; typhoid vaccine, live | Drugs.com |
| Alcohol/Food Interaction(s) | Fortaz® contains about 53 mg of sodium per gram of ceftazidime activity. This sodium content should be taken into consideration when treating patients who have conditions, such as congestive heart failure, hypertension (high blood pressure), or fluid retention, which require sodium-restricted diets. | Drugs.com |
| Disease Interaction(s) | Pseudomembranous colitis (major); Renal Dysfunction (moderate); Hemodialysis (moderate); Liver disease (moderate); Seizure disorders (moderate) | Drugs.com |
| On-target Side Effects | Red streaks on the skin; swelling, tenderness, or pain at injection site | Drugs.com |
| Off-target Side Effects | Rash, nausea, vomiting, diarrhea, abdominal pain, headache, dizziness, fever, jaundice, vaginitis, superinfection, anaphylaxis | Drugs.com |
| CYP Interactions | CYP450 3A4 substrate | DrugBank |
Ceftazidime is generally a well-tolerated antibiotic. The most common side effects reported in clinical trials were local reactions following IV administration in addition to allergic and gastrointestinal reactions (FDA, 2017). However, the recommended daily dosage should be reduced when ceftazidime is administered to patients with renal impairment (FDA, 2017). Elevated ceftazidime levels in such patients can result in adverse reactions, such as seizures, encephalopathy, coma, neuromuscular excitability, and myoclonia (FDA, 2017).
If the bacterial infection does not respond to monotherapy with ceftazidime, an aminoglycoside or similar antibacterial agent should be considered (FDA, 2017). However, renal function should be closely monitored if ceftazidime is concomitantly administered with high doses of aminoglycoside antibiotics because of the increased potential of nephrotoxicity (toxicity in the kidneys) and ototoxicity (toxicity to the ear) of aminoglycosides (FDA, 2017).
Cases of Clostridium difficile associated diarrhea (CDAD) have been reported with the use of almost all antibacterial drugs, including ceftazidime, and may vary in severity from mild diarrhea to fatal colitis. CDAD occurs because treatment with antibiotics changes the normal flora of the colon, which results in an overgrowth of C. difficile (FDA, 2017). If CDAD is suspected or confirmed, ongoing antibiotic therapy not targeted against C. difficile may need to be discontinued. It is also important to note that CDAD has been reported to occur even two months after antibiotic use (FDA, 2017).
Regulatory Approvals/Commercial
Ceftazidime was approved for medical use by the US FDA on July 19th, 1985 and is currently sold under the commercial name Fortaz® among other brand names. Fortaz® is indicated in the treatment of the following (FDA, 2017):
- Lower respiratory tract infections (including pneumonia)
- Skin and skin-structure infections
- Urinary tract infections (both complicated and uncomplicated)
- Bacterial septicemia
- Bone and joint infections
- Gynecologic infections (including endometritis and pelvic cellulitis)
- Intra-abdominal infections (including peritonitis)
- Central nervous system infections (including meningitis)
Fortaz® is available for intravenous or intramuscular use as a dry-powdered mixture of ceftazidime pentahydrate and sodium carbonate. The total sodium content of the powder is approximately 54 mg per gram of ceftazidime activity (FDA, 2017). The mixture must be combined with an appropriate diluent prior to administration. The recommended adult dosage is 1 g administered either intravenously or intramuscularly every 8-12 hours (FDA, 2017). Nevertheless, the dosage and route (IV or IM) should be determined by the severity of the infection, susceptibility of the pathogenic bacterial strain, and overall condition and renal function of the patient (FDA, 2017).
Links
Table 5. Links to learn more about ceftazidime
| Comprehensive Antibiotic Resistance Database (CARD) | ARO: 0000060 |
| DrugBank | DB00438 |
| Drugs.com | https://www.drugs.com/mtm/ceftazidime-injection.html |
| FDA | https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050578s061,050634s028lbl.pdf |
| LiverTox: National Institutes of Health (NIH) | https://www.ncbi.nlm.nih.gov/books/NBK548666/ |
| PubChem ID | 5481173 |
Learn about ceftazidime resistance.
References
Ceftazidime for injection. (2017) Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050578s061,050634s028lbl.pdf
Ceftazidime. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/5481173
Ceftazidime - DrugBank. Drugbank.ca https://go.drugbank.com/drugs/DB00438
Ceftazidime (injection). Drugs.com https://www.drugs.com/mtm/ceftazidime-injection.html
Han, S., Zaniewski, R.P., Marr, E.S., Lacey, B.M., Tomaras, A.P., Evdokimov, A., Miller, J.R., Shanmugasundaram, V. (2010) Structural basis for effectiveness of siderophore-conjugated monocarbams against clinically relevant strains of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 107, 22002-7. https://doi.org/10.1073/pnas.1013092107
Jia, B., Raphenya, A. R., Alcock, B., Waglechner, N., Guo, P., Tsang, K. K., Lago, B. A., Dave, B. M., Pereira, S., Sharma, A. N., Doshi, S., Courtot, M., Lo, R., Williams, L. E., Frye, J. G., Elsayegh, T., Sardar, D. Westman, E. L., Pawlowski, A. C., Johnson, T. A., Brinkman, F. S., Wright, G. D., and McArthur, A. G. (2017) CARD 2017: expansion and model-centric curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Research 45, D566-573. https://doi.org/10.1093/nar/gkw1004
LiverTox - Clinical and Research Information on Drug-Induced Liver Injury. National Institutes of Health. https://www.ncbi.nlm.nih.gov/books/NBK548666/
Sainsbury, S., Bird, L., Rao, V., Shepherd, S. M., Stuart, D. I., Hunter, W. N., Owens, R. J., Ren, J. (2011) Crystal structures of penicillin-binding protein 3 from Pseudomonas aeruginosa: comparison of native and antibiotic-bound forms. J Mol Biol. 405, 173-84. https://doi.org/10.1016/j.jmb.2010.10.024
March 2025, Gauri Patel, Helen Gao, Shuchismita Dutta; Reviewed by Dr. Andrew Lovering
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/cellwall-biosynth/pbp/blm/ceftazidime