Trimethoprim
Drug Name
Trimethoprim is a synthetic antibiotic with activity against most gram-positive aerobic cocci and some gram-negative aerobic bacilli. The drug binds to dihydrofolate reductase in susceptible bacteria, inhibiting tetrahydrofolic acid synthesis (Gleckman et al., 1981). Trimethoprim is often used in a synergistic combination with sulfamethoxazole to treat urinary tract infections, traveler’s diarrhea, middle ear infections, and bronchitis. When used with sulfamethoxazole, the drugs produce a bactericidal effect.
Table 1. Basic profile of trimethoprim.
| Description | Broad-spectrum diaminopyrimidine antibiotic |
| Target(s) | Dihydrofolate reductase |
| Generic | Trimethoprim, trimethoprim-sulfamethoxazole (TMP-SMX) combination |
| Commercial Name | Primsol, Sulfatrim (TMP-SMX), Bactrim (TMP-SMX), Septra (TMP-SMX) |
| Combination Drug(s) | Sulfamethoxazole, rifampicin, isoniazid |
| Other Synonyms | TMP |
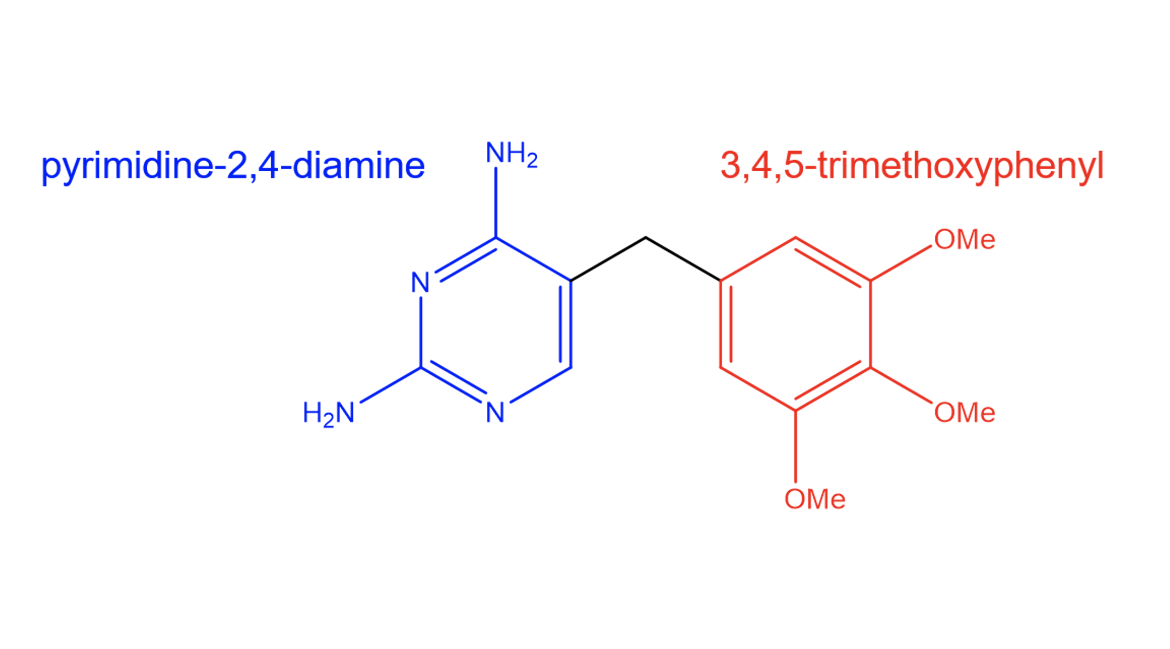
| IUPAC Name | 5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-diamine |
| Ligand Code in PDB | TOP |
| PDB Structure | 2w9g (Trimethoprim Bound to Target Protein) |
| ATC code | J01EA01 |
|
|
|
Antibiotic Chemistry
The antibacterial properties of trimethoprim can be attributed to its structural features (Figure 2). The drug has a diaminopyrimidine core which enables it to form hydrogen bonds with dihydrofolate reductase (DHFR). The structure of the drug also allows for van der Waals interactions with hydrophobic residues.
Drug Information
Table 2. Chemical and physical properties (DrugBank).
| Chemical Formula | C14H18N4O3 |
| Molecular Weight | 290.318 g/mol |
| Calculated Predicted Partition Coefficient: cLogP | 0.91 |
| Calculated Predicted Aqueous Solubility: cLogS | -2.86 |
| Solubility (in water) | 0.4 mg/mL |
| Predicted Topological Polar Surface Area (TPSA) | 105.51 Å2 |
Drug Target
Trimethoprim disrupts folate biosynthesis by inhibiting the enzyme dihydrofolate reductase. DHFR is responsible for the NADPH-dependent reduction of dihydrofolate to tetrahydrofolate. Metabolites of tetrahydrofolate serve as cofactors for the synthesis of protein and DNA precursors that are required for cell growth (Khavrutskii et al., 2007). Trimethoprim binds with high affinity to the active site of DHFR, preventing tetrahydrofolate synthesis. As a result, bacteria treated with trimethoprim are unable to synthesize new DNA or proteins because they lack tetrahydrofolate which leads to cell death.
Learn more about folate synthesis and DHFR.
Drug-Target Complex
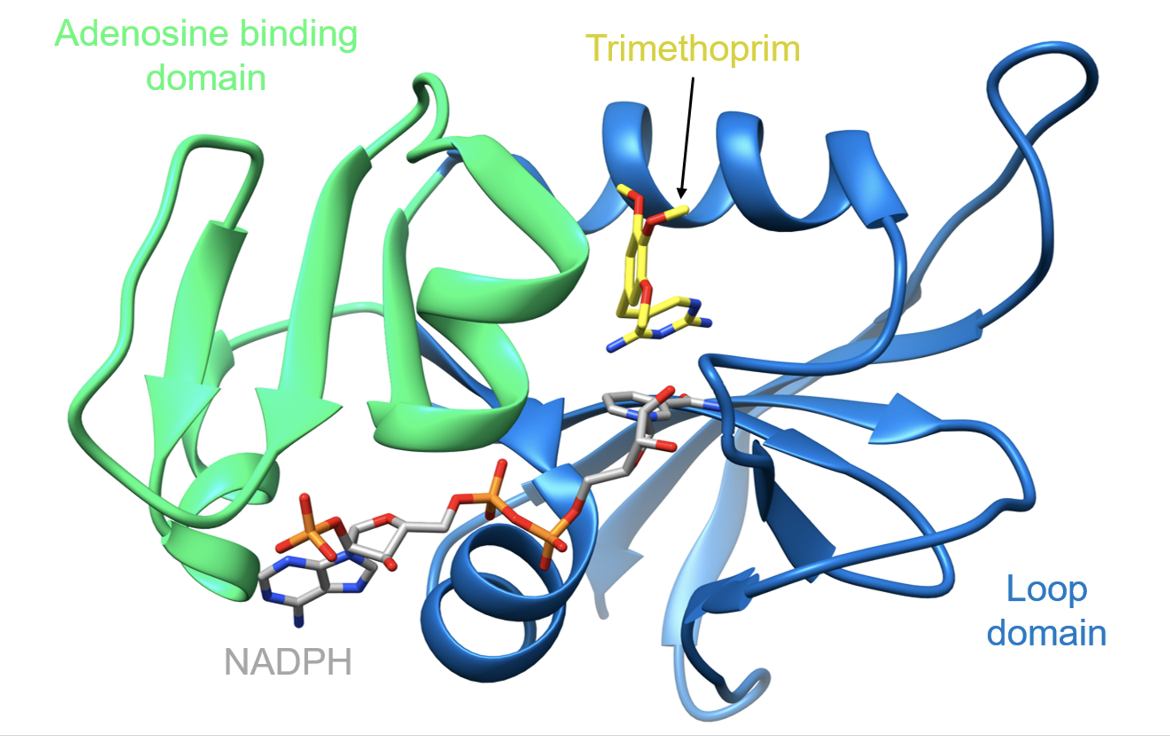
The target of trimethoprim, DHFR, consists of two domains which form a cleft for the active site (Sawaya and Kraut, 1997):
* Adenosine binding domain (residues 38-88, colored green)
* Loop domain (residues 1-37 and 89-159, colored blue)
|
| Figure 3. Ribbon representation of the wild-type Staphylococcus aureus DHFR in complex with trimethoprim and NADPH (PDB ID: 2w9g, Heaslet et al., 2009). |
Trimethoprim is a selective, competitive inhibitor of bacterial DHFR. Binding of trimethoprim in the folate-binding site buries 87% of its exposed surface area. This prevents dihydrofolate from binding to DHFR which blocks the synthesis of tetrahydrofolic acid.
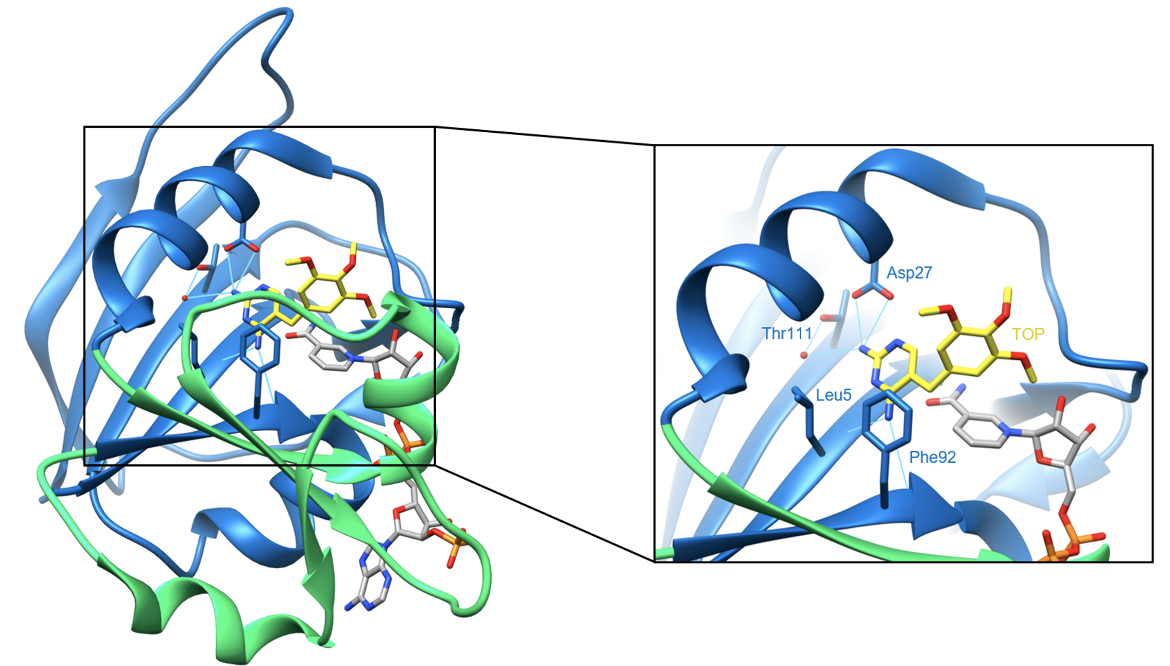
After entering the cell, trimethoprim binds in a large hydrophobic pocket within the active site known as the folate-binding site. The diaminopyrimidine (DAP) core of the drug forms four direct hydrogen bonds with DHFR residues. The side chain of Asp27 forms two hydrogen bonds with trimethoprim, while the main chains of Leu5 and Phe92 each form one hydrogen bond. There are two additional water-mediated hydrogen bonds between Thr111 and the drug (Heaslet et al., 2009). These hydrogen bonds are shown below in Figure 4.
|
| Figure 4. Ribbon representation of hydrogen bond interactions between trimethoprim and DHFR residues. Hydrogen bonds are colored in cyan (PDB ID: 2w9g, Heaslet et al., 2009). |
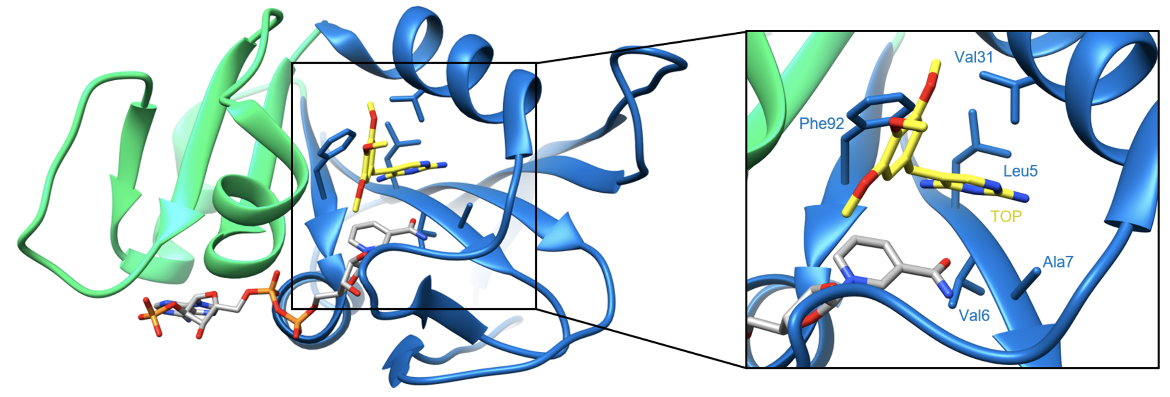
Trimethoprim affinity to DHFR is greatly improved when NADPH is bound in the active site. In S. aureus, the potency of trimethoprim is increased 400-fold in the presence of NADPH (Heaslet et al., 2009). Similar observations have been made in other species of bacteria including Escherichia coli (Baccanari et al., 1981). Despite their close proximity in DHFR, trimethoprim and NADPH do not form any hydrogen bonds. Instead, a series of van der Waals interactions are formed between the drug and the nicotinamide ring of NADPH. This appears to promote trimethoprim binding to DHFR. The DAP core of the drug also makes favorable van der Waals interactions with Leu5, Val6, Ala7, Val31, and Phe92 which further stabilizes trimethoprim-DHFR binding. These interactions can be visualized below in Figure 5.
|
| Figure 5. Ribbon representation of van der Waals interactions between trimethoprim and DHFR residues (PDB ID: 2w9g, Heaslet et al., 2009). |
Pharmacologic Property and Safety
Table 3. Pharmacokinetics: ADMET of trimethoprim.
| Features | Comment(s) | Source |
|---|---|---|
| Oral Bioavailability (%) | 100% (TMP-SMX) | FDA |
| IC50 | 20.4 nM (for E. coli) | (Cammarata et al., 2017) |
| Ki (nM) | 0.165 (for E. coli) | (Cammarata et al., 2017) |
| Half-Life (hrs) | 8-10 hours | DrugBank |
| Duration of Action | N/A | N/A |
| Absorption Site | N/A | N/A |
| Transporter(s) | N/A | N/A |
| Metabolism | The majority of trimethoprim metabolism involves the CYP2C9 and CYP3A4 enzymes | DrugBank |
| Excretion | ~50-60% of the drug is excreted in the urine within 24 hours, with approximately 80% of it being unchanged | DrugBank |
| AMES Test (Carcinogenic Effect) | Probability 0.8227 | DrugBank |
| hERG Safety Test (Cardiac Effect) | Probability 0.9394 | DrugBank |
| Liver Toxicity | Trimethoprim can cause idiosyncratic, acute liver injury 2-12 weeks after treatment | LiverTox |
Drug Interactions and Side Effects
Before starting treatment with trimethoprim, patients should inform their healthcare provider if they have any conditions such as anemia, folate deficiency, liver or kidney disease, or a blood disorder. A summary of possible drug interactions is listed in Table 4.
Table 4. Drug interactions and side effects of trimethoprim.
| Features | Comment(s) | Source |
|---|---|---|
| Total Number of Drug Interactions | 102 drugs | Drugs.com |
| Major Drug Interactions | 41 major drug interactions (e.g., dofetilide, irbesartan, methotrexate) | Drugs.com |
| Alcohol/Food Interactions | Do not drink alcohol while taking trimethoprim. There may be unpleasant side effects such as fast heartbeats, nausea, and vomiting. | Drugs.com |
| Disease Interactions | 3 disease interactions (folate deficiency, renal dysfunction, and dialysis) | Drugs.com |
| On-Target Side Effects | The most commonly reported side effect is a skin rash. Less common side effects include joint/muscle pain, nausea, neck stiffness, and changes in facial skin color. | Drugs.com |
| Off-Target Side Effects | N/A | N/A |
| CYP Interactions | CYP2C9, CYP3A4, CYP1A2 substrates | DrugBank |
In addition to targeting bacteria DHFR, Trimethoprim can bind to human DHFR. However, the binding affinity of trimethoprim for bacterial dihydrofolate reductase is several thousand times greater than its affinity for human dihydrofolate reductase. The active sites of the pathogen and human DHFR also differ. So when the drug is prescribed, it is more likely to target and inhibit bacterial DHFR. Still, drugs that target human DHFR, such as the anticancer agent methotrexate, can cause major drug interactions when given in conjunction with trimethoprim.
Regulatory Approvals/Commercial
Trimethoprim was first used in 1962 and was later registered for clinical use in combination with sulfonamides in 1968 (Huovinen et al., 1995). Today, a combination of trimethoprim and sulfamethoxazole is a commonly used synergistic antimicrobial combination. It is on the World Health Organization’s list of essential medicines and is available as a low-cost drug. It has received US FDA approval (FDA, 2016) for:
1. Urinary tract infections
2. Traveler’s diarrhea (in adults only)
3. Shigellosis
4. Acute infective exacerbation of chronic bronchitis
5. Otitis media
6. Pneumocystis carinii pneumonia
Trimethoprim is available as a tablet and the trimethoprim-sulfamethoxazole combination as a tablet and suspension. In both dosage forms for the combination, a 1:5 ratio of trimethoprim to sulfamethoxazole is used. This diminishes possible side effects of inhibiting human DHFR by trimethoprim (Fernández-Villa et al., 2019).
Links
Table 5: Links to learn more about trimethoprim
| Comprehensive Antibiotic Resistance Database (CARD) | ARO: 3000188 |
| DrugBank | DB00440 |
| Drugs.com | https://www.drugs.com/mtm/trimethoprim.html |
| FDA – TRIMETHOPRIM | https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018679s044lbl.pdf |
| LiverTox: National Institutes of Health (NIH) | https://www.ncbi.nlm.nih.gov/books/NBK547937/ |
| PubChem CID | 5578 |
Learn about Trimethoprim Resistance.
References
Baccanari, D.P., Stone, D., Kuyper, L. (1981). Effect of a single amino acid substitution on Escherichia coli dihydrofolate reductase catalysis and ligand binding. J. Biol. Chem. 256:1738–1747.
Bactrim. (2013). Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/017377s068s073lbl.pdf
Burchall J.J., Hitchings G.H. (1965). Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacology.1(2), 126–136.
Cammarata, M., Thyer, R., Lombardo, M., Anderson, A., Wright, D., Ellington, A., Brodbelt, J. (2017). Characterization of trimethoprim resistant E. coli dihydrofolate reductase mutants by mass spectrometry and inhibition by propargyl-linked antifolates. Chemical Science, 8(5), 4062-4072. doi:10.1039/c6sc05235e
Fernández-Villa, D., Aguilar, M. R., Rojo, L. (2019). Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications. International Journal of Molecular Sciences, 20(20), 4996. https://doi.org/10.3390/ijms20204996
Gleckman, R., Blagg, N., Joubert, D. (1981). Trimethoprim: Mechanisms of Action, Antimicrobial Activity, Bacterial Resistance, Pharmacokinetics, Adverse Reactions, and Therapeutic Indications. Pharmacotherapy: The Journal Of Human Pharmacology And Drug Therapy, 1(1), 14-19. doi:10.1002/j.1875-9114.1981.tb03548.x
Heaslet, H., Harris, M., Fahnoe, K., Sarver, R., Putz, H., Chang, J. et al. (2009). Structural comparison of chromosomal and exogenous dihydrofolate reductase from Staphylococcus aureus in complex with the potent inhibitor trimethoprim. Proteins: Structure, Function, And Bioinformatics, 76(3), 706-717. https://doi.org/10.1002/prot.22383 PDB ID: 2w9g
Huovinen, P., Sundstrom, L., Swedberg, G., Skold, O. (1995). Trimethoprim and sulfonamide resistance. Antimicrobial Agents And Chemotherapy, 39(2), 279-289. https://doi.org/10.1128/aac.39.2.279
Khavrutskii, I. V., Price, D. J., Lee, J., Brooks, C. L. (2007). Conformational Change of the Methionine 20 loop of Escherichia coli Dihydrofolate Reductase Modulates pKa of the Bound Dihydrofolate. Protein Science: A Publication of the Protein Society, 16(6), 1087–1100. https://doi.org/10.1110/ps.062724307
LiverTox – Clinical and Research Information on Drug-induced Liver Injury. National Institutes of Health. https://www.ncbi.nlm.nih.gov/books/NBK547937/
Trimethoprim. Drugs.com. https://www.drugs.com/mtm/trimethoprim.html
Trimethoprim– DrugBank. Drugbank.ca. https://www.drugbank.ca/drugs/DB00440
Trimethoprim. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Trimethoprim
Trimethoprim Tablets, USP. (2016). Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018679s044lbl.pdf
March 2025, Steven Arnold, Shuchismita Dutta; Reviewed by Dr. Christina Bourne
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/folate-synth/DHR/diaminopyrimidine/trimethoprim