Folate Synthesis
Introduction
Folate is an essential micronutrient in all living organisms. It takes part in one-carbon transfer enzymatic reactions and is critical for syntheses of certain amino acids (e.g., methionine), purine nitrogenous bases, thymine. Folate also plays a key role in the epigenetic regulation of gene expression (via methylation). As a result, all organisms must have a source of folate to grow, develop, and survive. Humans are not able to synthesize folate de novo and must obtain it via dietary supplementation (Fernández-Villa et al., 2019). Folate is present in several foods such as fruits, fortified cereals, and green leafy vegetables. In humans, folate deficiency is linked to an increased risk of developing neural tube defects, cardiovascular disease, and cancer (Scaglione anda Panzavolta, 2013).
Unlike humans, bacteria cannot obtain folate via dietary sources. Bacterial cell walls lack folate receptors to transport folate into the cell, so bacteria contain specific enzymes that synthesize folate de novo (Fernández-Villa et al., 2019). For this reason, these enzymes are attractive targets for developing new antibacterial agents.
Folate, Folic Acid, and Tetrahydrofolate
Folate is a generic term describing a family of compounds including folic and its derivatives. Herein we define specific folate compounds and the roles they play in biology.
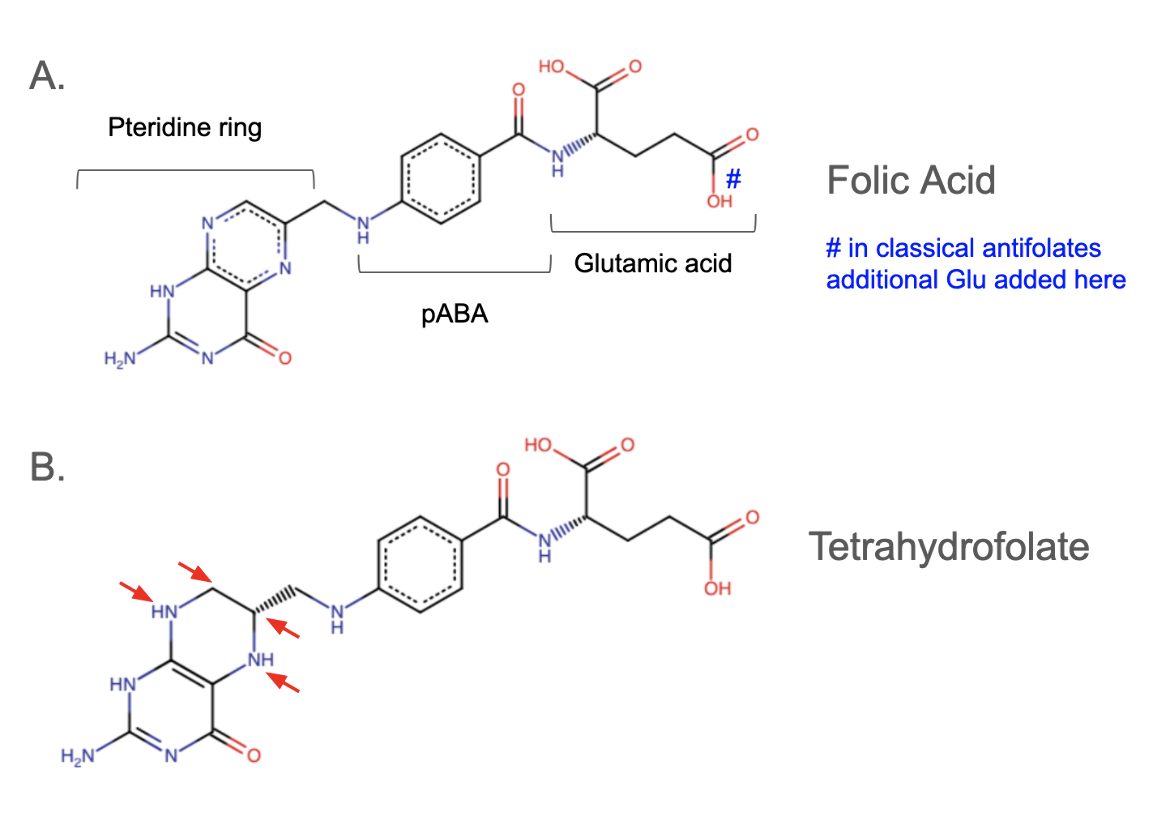
Folic acid is a synthetic molecule and the parent compound of this family of molecules. It belongs to the vitamin-B complex family, a group of small water-soluble molecules that serve as cofactors for a range of metabolic functions (Scaglione and Panzavolta, 2013). It contains three chemical components: a pteridine ring, pABA, and glutamic acid (see Figure 1a). Folic acid itself is not active as a coenzyme and must be converted into a metabolically active form called tetrahydrofolate or THF (Tjong et al., 2024).
All living cells use THF to metabolize of certain amino acids, purines, and thymine (Tjong et al., 2024). Folic acid is converted to THF by the addition of four hydrogen atoms to 5,6,7 and 8 positions in the pteridine ring. The enzyme dihydrofolate reductase catalyzes this reaction in the presence of the cofactor NADPH (nicotinamide adenine dinucleotide phosphate), which serves as an electron donor. The chemical structure of THF is shown below in Figure 1b.
Folate Synthesis Pathway
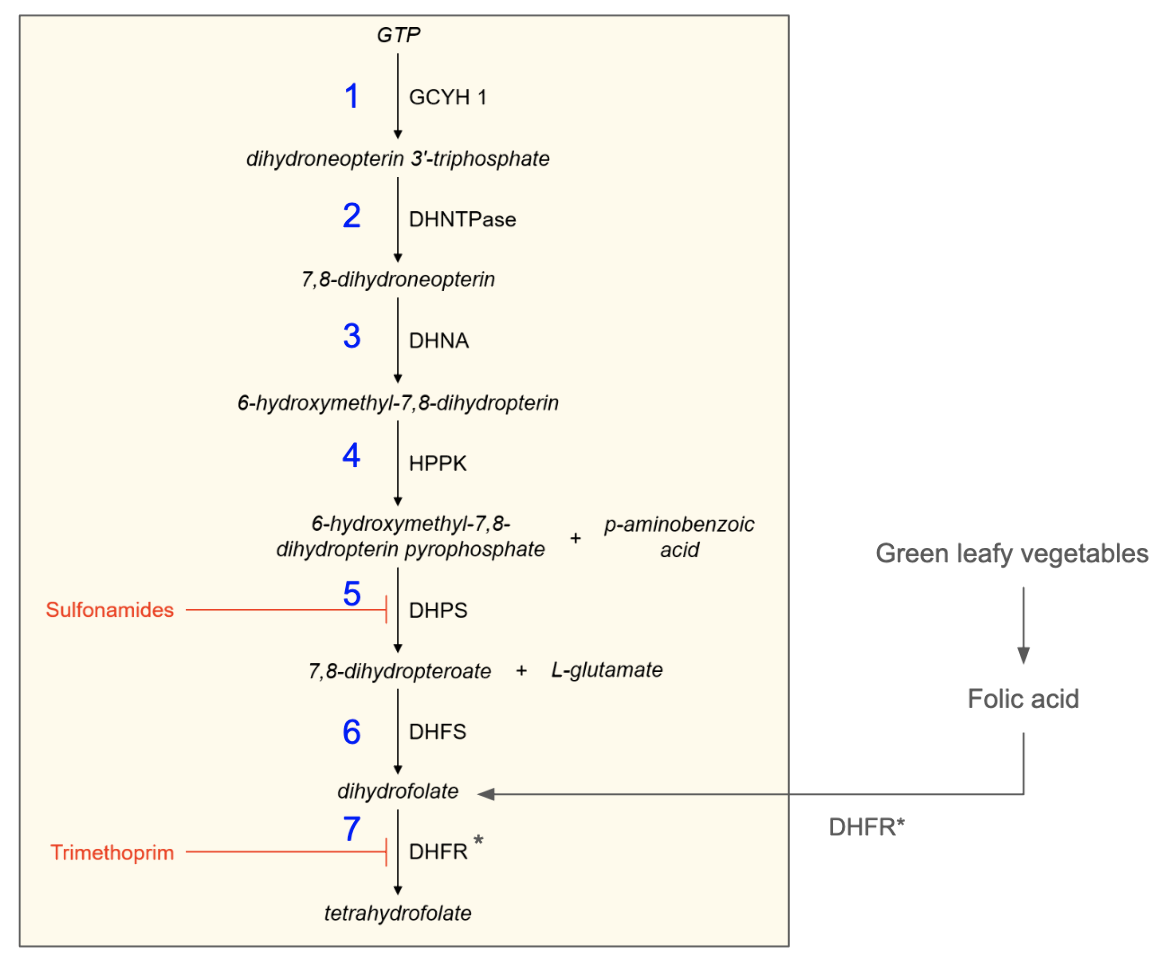
The THF synthesis pathway in bacteria is complex, involving seven enzymes and three precursors - GTP, p-aminobenzoic acid (or pABA), and glutamate (Bourne, 2014). Key steps in this biosynthetic pathway are listed below and summarized in Figure 2.
1. Guanine cyclohydrolase (GCYH 1) catalyzes the conversion of GTP to yield dihydroneopterin 3′-triphosphate.
2. Dihydroneopterin hydrolase (DHNTPase) catalyzes the conversion of dihydroneopterin 3′-triphosphate to yield 7,8-dihydroneopterin.
3. Dihydroneopterin aldolase (DHNA) catalyzes the conversion of 7,8-dihydroneopterin to yield 6-hydroxymethyl-7,8-dihydropterin.
4. Hydroxymethyl-dihydropteridine pyrophosphokinase (HPPK) catalyzes the conversion of 6-hydroxymethyl-7,8-dihydropterin to yield 6-hydroxymethyl-7,8-dihydropterin pyrophosphate (using ATP as a cofactor).
5. Dihydropteroate synthase (DHPS) catalyzes the conversion of 6-hydroxymethyl-7,8-dihydropterin pyrophosphate with para-aminobenzoic acid to yield 7,8-dihydropteroate.
6. Dihydrofolate synthase (DHFS) catalyzes the conversion of 7,8-dihydropteroate with L-glutamate to yield dihydrofolate (using ATP as a cofactor).
7. Dihydrofolate reductase (DHFR) catalyzes the conversion of dihydrofolate to yield tetrahydrofolate (using NADPH as a cofactor).
Steps 1-6 are exclusive to bacteria, leading up to the synthesis of dihydrofolate (DHF). Humans convert folic acid (obtained from the diet) into DHF via the action of DHFR (shown outside the yellow shaded box in Figure 2). DHFR catalyzes the conversion of Folic acid into DHF. Step 7 - i.e., conversion of DHF into tetrahydrofolate (THF) is similar among bacteria and humans. The DHFR-catalyzed steps are NADPH-dependent. Thus the synthesis of THF in bacteria has one NADPH-dependent reaction while that in humans uses folic acid and requires two sequential NADPH-dependent reactions (Wright and Anderson, 2011).
Learn more about DHPS.
Learn more about DHFR.
Folate Synthesis as a Target of Antibiotics
The role of folate in bacterial survival is well established - without a source of folate, bacteria die. Folate synthesis can be interdicted by targeting enzymes within the cytoplasm. The chemical structures of approved antibiotics that inhibit intracellular enzymes involved in folate synthesis are diverse. Folate inhibitors may be organized into two groups:
* “classical antifolates” are structural analogues of folic acid and contain a polyglutamate moiety. These additional glutamates are linked to the gamma carboxyl group of the glutamate group via peptide bonds (see Figure 1A) and tend to be negatively charged which limits their ability to diffuse across cell membranes. In eukaryotic cells, the polyglutamate moiety is critical for engaging with receptors for its uptake.
* “nonclassical antifolates” are molecules where the pteridine moiety (pictured in Figure 1) is replaced with a 2,4-aminopyrimidine. They are structurally diverse from folate (Kompis et al., 2005). All FDA approved antibacterial agents that target folate synthesis are classified as nonclassical antifolates (Fernández-Villa et al., 2019).
The most important antibiotics used in clinical settings are trimethoprim and sulfamethoxazole. Trimethoprim competitively inhibits DHFR and sulfamethoxazole competitively inhibits DHPS. These two drugs are frequently prescribed together in a synergistic combination to treat various types of bacterial infections (Fernández-Villa et al., 2019).
References
Bourne C. R. (2014). Utility of the Biosynthetic Folate Pathway for Targets in Antimicrobial Discovery. Antibiotics (Basel, Switzerland), 3(1), 1–28. https://doi.org/10.3390/antibiotics3010001
Fernández-Villa, D., Aguilar, M. R., Rojo, L. (2019). Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications. International Journal of Molecular Sciences, 20(20), 4996. https://doi.org/10.3390/ijms20204996
Kompis, I., Islam, K., Then, R. (2005). DNA and RNA Synthesis: Antifolates. Chemical Reviews, 105 (2), 593-620 36(24). https://pubs.acs.org/doi/10.1021/cr0301144
Scaglione, F., Panzavolta, G. (2014). Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica, 44(5), 480-488. https://doi.org/10.3109/00498254.2013.845705
Tjong, E., Dimri, M., Mohiuddin, S.S. (2024), Biochemistry, Tetrahydrofolate. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, https://www.ncbi.nlm.nih.gov/books/NBK539712/
Wright, D. L., Anderson, A. C. (2011). Antifolate agents: a patent review (2006 - 2010). Expert Opinion on Therapeutic Patents, 21(9), 1293–1308. https://doi.org/10.1517/13543776.2011.587804
March 2025, Steven Arnold, Shuchismita Dutta; Reviewed by Dr. Christina Bourne
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/folate-synth