Sulfamethoxazole
Drug Name
Sulfamethoxazole is a synthetic sulfonamide antibiotic with activity against gram-positive and gram-negative bacteria. The drug binds to dihydropteroate synthase in susceptible bacteria, inhibiting tetrahydrofolic acid synthesis (Fernández-Villa et al., 2019). Sulfamethoxazole is often used in a synergistic combination with trimethoprim to treat urinary tract infections, traveler’s diarrhea, middle ear infections, and bronchitis. When used with trimethoprim, the drugs produce a bactericidal effect.
Table 1. Basic profile of These concentrations may not be the latest values approved by US FDA.
| Description | Intermediate-acting sulfonamide antibiotic |
| Target(s) | Dihydropteroate synthase |
| Generic | Sulfamethoxazole, trimethoprim-sulfamethoxazole combination (TMP-SMX) |
| Commercial Name | Bactrim (TMP-SMX), Sulfatrim (TMP-SMX), Septra (TMP-SMX) |
| Combination Drug(s) | Bactrim (Sulfamethoxazole and trimethoprim) |
| Other Synonyms | SMX |
| IUPAC Name | 4-amino-N-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide |
| Ligand Code in PDB | 08D |
| PDB Structure | 3tzf (Sulfamethoxazole Bound to Target Protein) |
| ATC code | J01EC01 |
|
|
|
Antibiotic Chemistry
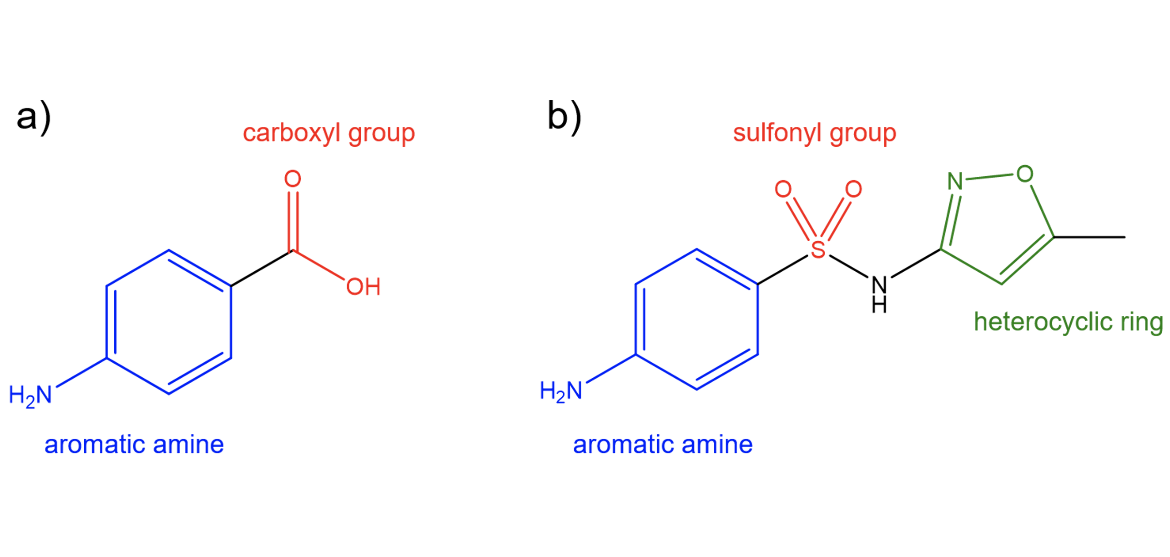
Sulfamethoxazole is a structural analog of pABA, the natural substrate for dihydropteroate synthase (DHPS). Both molecules contain an aromatic amine attached to an electron-withdrawing group. pABA features a carboxyl group, while sulfamethoxazole contains a sulfonyl group attached to a heterocyclic ring. Sulfamethoxazole uses its structural similarity with pABA to bind to the pABA-binding pocket and competitively inhibit DHPS.
|
| Figure 2. (a) Chemical structure of p-aminobenzoic acid (pABA). (b) Chemical structure of sulfamethoxazole. Structures created using ChemDraw. |
Drug Information
Table 2. Chemical and physical properties (DrugBank).
| Chemical Formula | C10H11N3O3S |
| Molecular Weight | 253.278 g/mol |
| Calculated Predicted Partition Coefficient (cLogP) | 0.89 |
| Calculated Predicted Aqueous Solubility (cLogS) | -2.62 |
| Solubility (in water) | 0.61 mg/mL |
| Predicted Topological Polar Surface Area (TPSA) | 98.22 Å2 |
Drug Target
Sulfamethoxazole disrupts tetrahydrofolate biosynthesis by inhibiting the enzyme dihydropteroate synthase. DHPS catalyzes a reaction between dihydropteroate pyrophosphate (DHPP) and p-aminobenzoic acid (pABA) to produce dihydropteroate. This product serves as the precursor to dihydrofolate, which is then reduced to tetrahydrofolate (Fernández-Villa et al., 2019).
Sulfamethoxazole is a direct competitor of pABA. Without pABA bound in the active site, DHPS is unable to synthesize dihydropteroate. Inhibition of this essential step means bacteria cannot synthesize new DNA or proteins, resulting in a bacteriostatic effect.
Learn more about folate synthesis and DHPS.
Drug-Target Complex
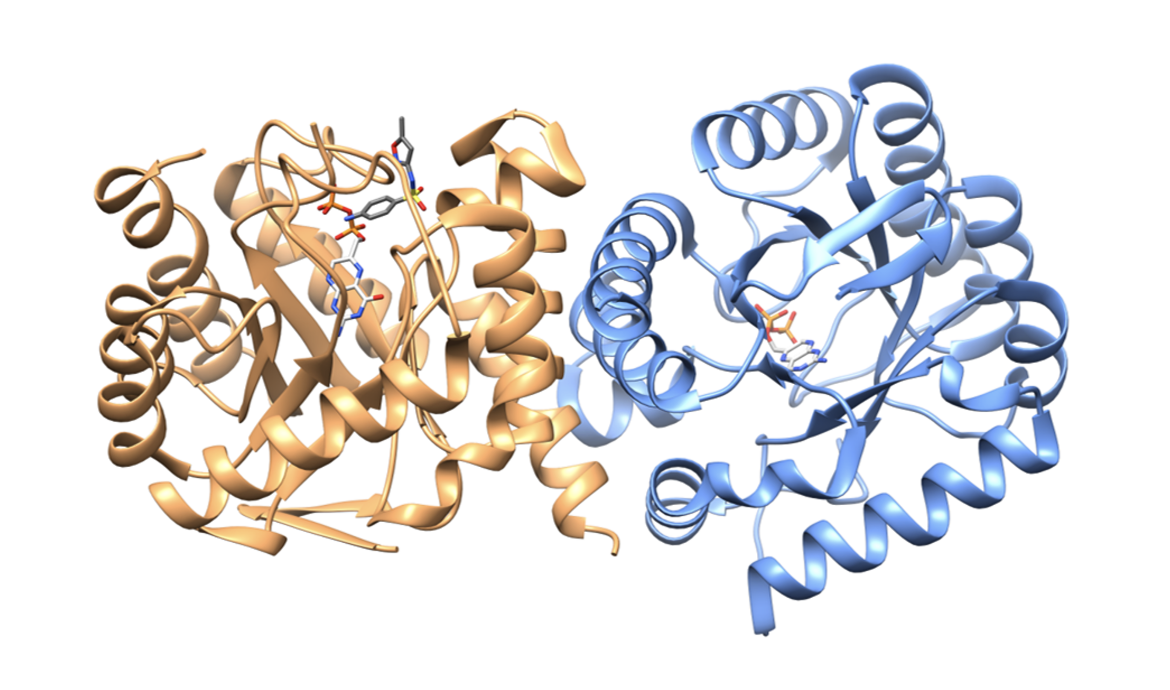
The structure of DHPS comprises a dimer in the asymmetric unit with each monomer composing around 300 residues. In most species, each monomer adopts a TIM barrel-type fold (Banner et al., 1975) in which repeating α/β units make an eight-stranded parallel β-barrel surrounded by eight α helices. There are two binding pockets in each monomer: one that binds DHPP and one that binds pABA (Babaoglu et al., 2004). Figure 3 shows the DHPS dimer with DHPP and sulfamethoxazole bound. For unknown reasons, sulfamethoxazole was only visible in one monomer (Yun et al., 2012).
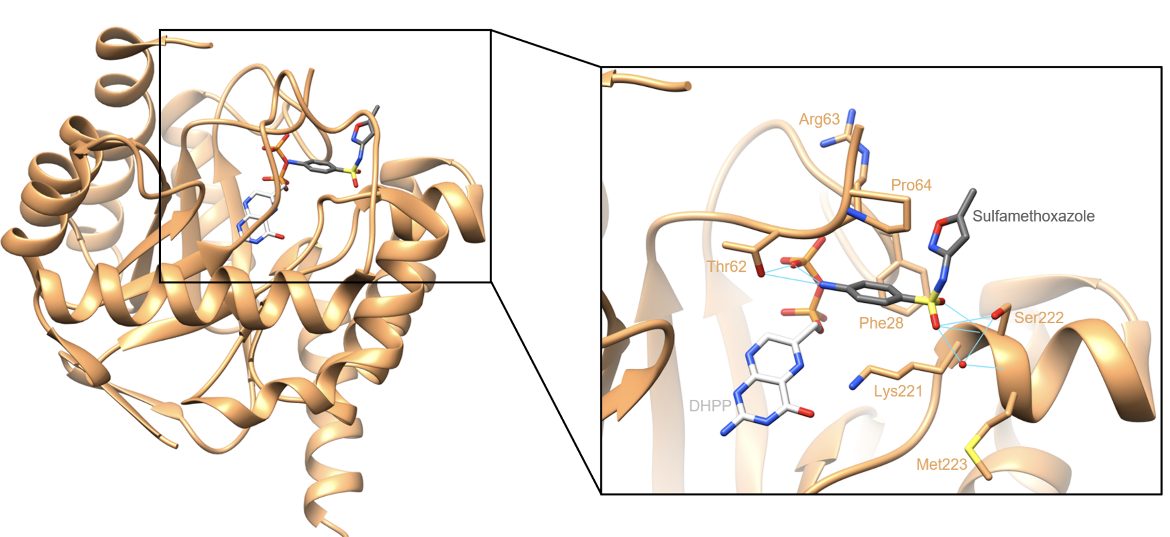
Sulfamethoxazole binds within the pABA binding pocket via hydrogen-bonding and hydrophobic interactions. The oxygen atoms in the sulfonyl group form direct hydrogen bonds with Ser222, and the amine attached to the aromatic ring makes a hydrogen bond with Thr62. Additionally, the sulfonyl group forms water-mediated hydrogen bonds with Ser222 and Met223. The drug also forms hydrophobic interactions with residues that line the pABA binding pocket, such as Phe28, Arg63, Pro64, and Lys221 (Yun et al., 2012).
Residues 66 and 67 were disordered in the crystal structure
These hydrogen-bonding and hydrophobic interactions can be seen below in the crystal structure in Figure 4. In this structure, DHPS was crystallized with DHPP and sulfamethoxazole. Since the DHPP and pABA binding pockets are close in proximity, sulfamethoxazole was observed to form a hydrogen bond with the pyrophosphate moiety of DHPP (Yun et al., 2012).
|
| Figure 4. Ribbon representation of the DHPS active site occupied by DHPP and sulfamethoxazole. Hydrogen bonds are colored in cyan. (PDB ID: 3tzf, Yun et al., 2012). |
Within the pABA binding pocket, sulfamethoxazole superimposes well with pABA. The negatively-charged oxygen atoms in the sulfonyl group match the carboxyl group of pABA, and the common phenyl groups lie in the same hydrophobic pocket (Yun et al., 2012). This illustrates how the drug uses its structural similarity with pABA to occupy the pABA binding pocket and competitively inhibit DHPS.
Pharmacologic Properties and Safety
Table 3. Pharmacokinetics: ADMET of sulfamethoxazole.
| Features | Comment(s) | Source |
|---|---|---|
| Oral Bioavailability (%) | 85-90% | DrugBank |
| IC50 | 2.7 μM (for T. gondii) | (Allegra et al., 1990) |
| Ki | 21 (for T. gondii) | (Allegra et al., 1990) |
| Half-Life (hrs) | 10 hours | DrugBank |
| Duration of Action | 1-4 hours after oral administration (for TMP-SMX) | FDA |
| Absorption Site | N/A | N/A |
| Transporter(s) | N/A | N/A |
| Metabolism | Sulfamethoxazole is primarily metabolized by arylamine N-acetyltransferase enzymes. It may also undergo oxidation or glucuronidation. | DrugBank |
| Excretion | Approximately 84.5% of a single dose is recovered in urine within 72 hours. Around 30% of this is the unchanged drug, and the remainder is the acetylated metabolite. | DrugBank |
| AMES Test (Carcinogenic Effect) | Probability 0.9132 | DrugBank |
| hERG Safety Test (Cardiac Effect) | Probability 0.9143 | DrugBank |
| Liver Toxicity | TMP-SMX can cause a characteristic idiosyncratic liver injury that has features of a drug-allergy or hypersensitivity. The typical onset is development of a rash or fever followed by jaundice. | LiverTox |
Drug Interactions and Side Effects
Before starting treatment with TMP-SMX, patients should inform their health care provider if they have any of the following conditions:
* Severe liver disease
* Anemia caused by folic acid deficiency
* Kidney disease that is not being monitored
* Currently taking dofetilide
Table 4. Drug interactions and side effects of sulfamethoxazole.
| Features | Comment(s) | Source |
|---|---|---|
| Total Number of Drug Interactions | 348 drugs | Drugs.com |
| Major Drug Interactions | 54 drugs (e.g., dofetilide, fosinopril, methotrexate) | Drugs.com |
| Alcohol/Food Interactions | Do not drink alcohol while taking TMP-SMX. There may be unpleasant side effects such as fast heartbeats, nausea, and vomiting. | Drugs.com |
| Disease Interactions | 12 disease interactions (e.g., colitis, renal dysfunction, liver disease) | Drugs.com |
| On-Target Side Effects | TMP-SMX may cause some unwanted side effects. Examples include nausea, loss of appetite, rashes, and vomiting. | Drugs.com |
| Off-Target Side Effects | N/A | N/A |
| CYP Interactions | CYP2C9 and CYP3A4 substrate | Drugs.com |
Cases of Clostridium difficile associated diarrhea (CDAD) have been reported with the use of almost all antibacterial drugs, including sulfamethoxazole, and may vary in severity from mild diarrhea to fatal colitis. CDAD occurs because treatment with antibiotics changes the normal bacterial flora of the colon, which results in an overgrowth of C. difficile (FDA, 2013).
Regulatory Approvals/Commercial
Sulfonamides were discovered in 1932 and put into clinical use in 1935. The development of sulfonamide derivatives, such as sulfamethoxazole, enabled them to be used extensively in different clinical indications (Eliopoulos and Huovinen, 2001).Today, trimethoprim and sulfamethoxazole is a commonly used synergistic antimicrobial combination. It is on the World Health Organization’s list of essential medicines, and is available as a low-cost drug. It has received US FDA approval for (FDA, 2013):
a. Urinary tract infections
b. Traveler’s diarrhea (in adults only)
c. Shigellosis
d. Acute infective exacerbation of chronic bronchitis
e. Otitis media
f. Pneumocystis carinii pneumonia
Sulfamethoxazole is available in a TMP-SMX combination as a tablet and suspension. In both dosage forms for the combination, a 1:5 ratio of trimethoprim to sulfamethoxazole is used. This diminishes possible side effects of inhibiting human dihydrofolate reductase by trimethoprim (Fernández-Villa et al., 2019).
Links
Table 5. Links to learn more about sulfamethoxazole
| Comprehensive Antibiotic Resistance Database (CARD) | ARO: 3000329 |
| DrugBank | DB01015 |
| Drugs.com | https://www.drugs.com/mtm/sulfamethoxazole-and-trimethoprim.html |
| FDA – BACTRIM | https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/017377s068s073lbl.pdf |
| LiverTox: National Institutes of Health (NIH) | https://www.ncbi.nlm.nih.gov/books/NBK547937/ |
| PubChem CID | 5329 |
Learn about sulfamethoxazole resistance.
References
Allegra, C. J., Boarman, D., Kovacs, J. A., Morrison, P., Beaver, J., Chabner, B. A., Masur, H. (1990). Interaction of sulfonamide and sulfone compounds with Toxoplasma gondii dihydropteroate synthase. The Journal of clinical investigation, 85(2), 371-379. https://doi.org/10.1172/JCI114448
Babaoglu, K., Qi, J., Lee, R., White, S. (2004). Crystal Structure of 7,8-Dihydropteroate Synthase from Bacillus anthracis. Structure, 12(9), 1705-1717. https://doi.org/10.1016/j.str.2004.07.011
Bactrim (sulfamethoxazole and trimethoprim) (2013) Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/017377s068s073lbl.pdf
Banner, D., Bloomer, A., Petsko, G., Phillips, D., Pogson, C., Wilson, I., Corran, P., Furth, A., Milman, J., Offord, R., Priddle, J., Waley, S. (1975). Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5Å resolution: using amino acid sequence data. Nature, 255(5510), 609-614. https://doi.org/10.1038/255609a0
Fernández-Villa, D., Aguilar, M. R., Rojo, L. (2019). Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications. International Journal of Molecular Sciences, 20(20), 4996. https://doi.org/10.3390/ijms20204996
Jia, B., Raphenya, A. R., Alcock, B., Waglechner, N., Guo, P., Tsang, K. K., Lago, B. A., Dave, B. M., Pereira, S., Sharma, A. N., Doshi, S., Courtot, M., Lo, R., Williams, L. E., Frye, J. G., Elsayegh, T., Sardar, D. Westman, E. L., Pawlowski, A. C., Johnson, T. A., Brinkman, F. S., Wright, G. D., McArthur, A. G. (2017) CARD 2017: expansion and model-centric curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Research 45, D566-573. https://doi.org/10.1093/nar/gkw1004
LiverTox - Clinical and Research Information on Drug-Induced Liver Injury. National Institutes of Health. https://www.ncbi.nlm.nih.gov/books/NBK547937/
Sulfamethoxazole. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/5329
Sulfamethoxazole - DrugBank. Drugbank.ca https://go.drugbank.com/drugs/DB01015
Sulfamethoxazole and trimethoprim. Drugs.com https://www.drugs.com/mtm/sulfamethoxazole-and-trimethoprim.html
Yun, M. K., Wu, Y., Li, Z., Zhao, Y., Waddell, M. B., Ferreira, A. M., Lee, R. E., Bashford, D., White, S. W. (2012) Catalysis and sulfa drug resistance in dihydropteroate synthase. Science, 335, 1110-4. https://doi.org/10.1126/science.1214641
January 2025, Steven Arnold, Helen Gao, Shuchismita Dutta; Reviewed by Dr. Christina Bourne
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/folate-synth/DHS/sulfonamide/sulfamethoxazole