Dihydropteroate Synthase
The first step in the de novo biosynthesis of folate is catalyzed by the enzyme Dihydropteroate synthase (DHPS). It is found in the cytoplasmic region of bacteria and is the target of the US FDA approved antibacterial drug sulfamethoxazole.
Function
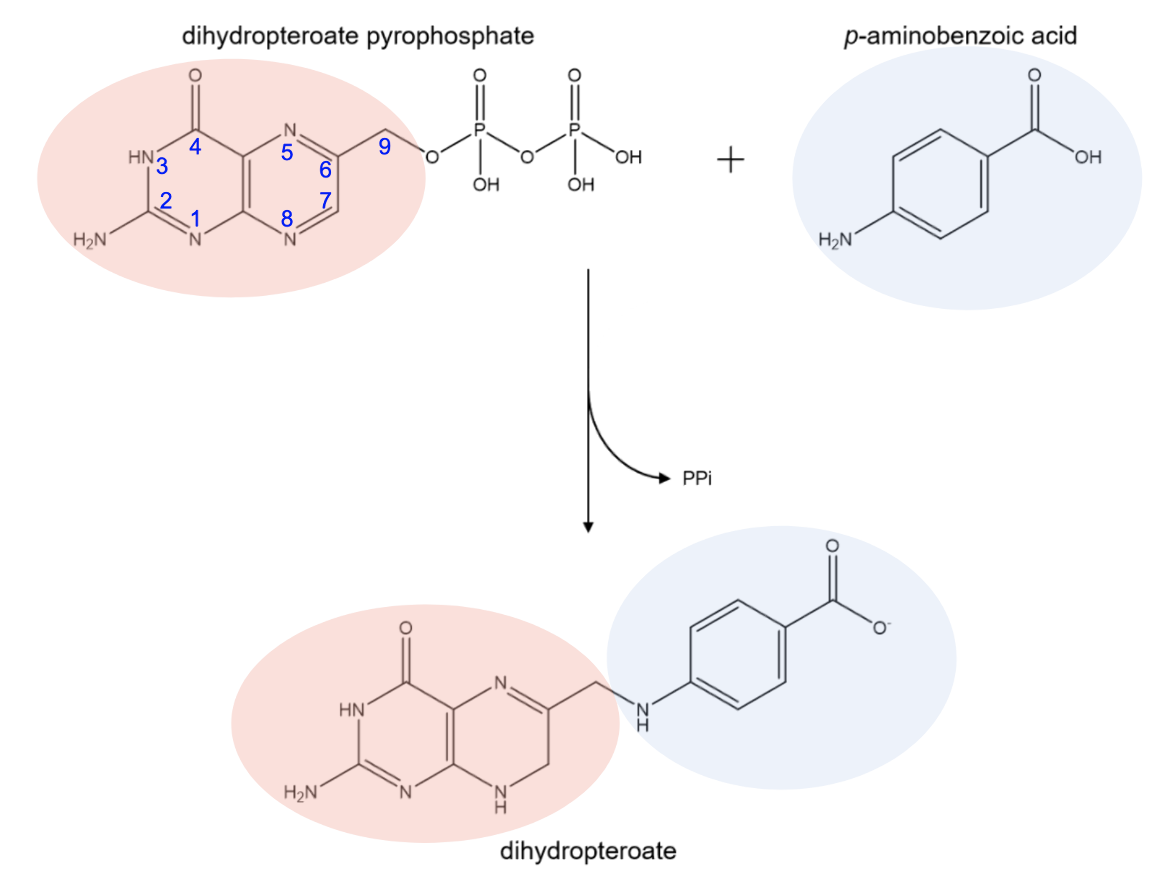
The enzyme DHPS is responsible for the synthesis of dihydropteroate. It contains two binding pockets: one that binds dihydropteroate pyrophosphate (DHPP) and one that binds p-aminobenzoic acid (pABA). DHPS catalyzes the formation of a bond between the C9 carbon of DHPP and the amino nitrogen of pABA to form dihydropteroate (Figure 1). This product serves as the precursor to dihydrofolate, which is then reduced to tetrahydrofolate. The pyrophosphate moiety (PPi) of DHPP is released during the reaction catalyzed by DHPS (Yun et al., 2012).
|
| Figure 1. Biosynthesis reaction of dihydropteroate catalyzed by DHPS. Chemical structures were created using ChemDraw. |
The formation of dihydropteroate is considered to be the first regulation point during the de novo biosynthesis of folate. Mammalian cells obtain folic acid from dietary sources, they do not have this enzyme. DHPS is present in bacteria since they do not have a transporter for the uptake of folates. Therefore this enzyme's activity is essential for the survival of microbes making it an attractive target of novel antimicrobial agents (Fernandez-Villa et al., 2019).
Structure
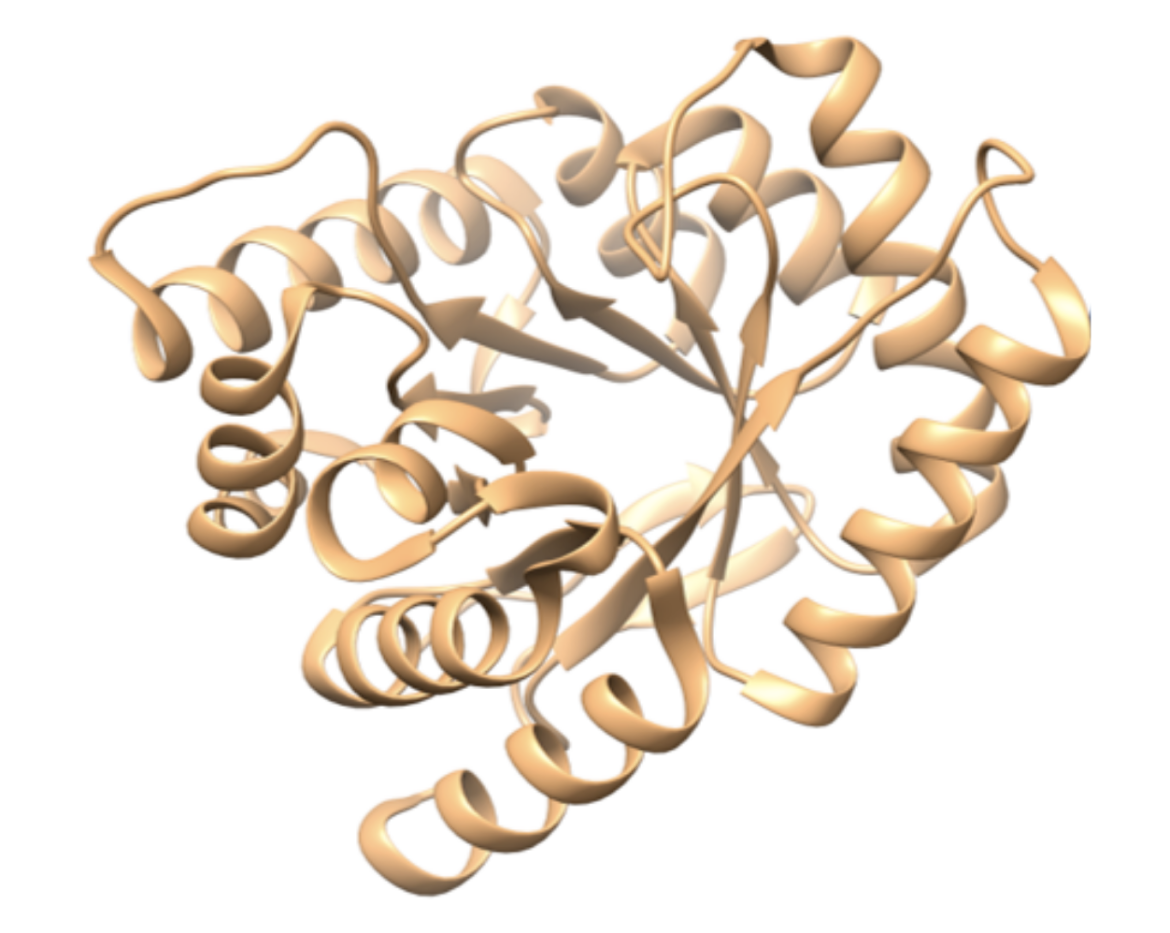
Although the asymmetric unit of the DHPS structure examined here contains two identical monomers of around 300 amino acids, here we show only one copy of the protein (Figure 2). The overall fold of the enzyme is similar among many species of bacteria, including B. anthracis, E. coli, S. aureus, and M. tuberculosis (Babaoglu et al., 2004). DHPS has a TIM barrel α/β structure (Banner et al., 1975) in which repeating α/β units make an eight-stranded parallel β-barrel surrounded by eight α helices.
|
| Figure 2. Ribbon representation of a monomer of the B. anthracis unliganded DHPS. (PDB ID: 1tws, Babaoglu et al., 2004). |
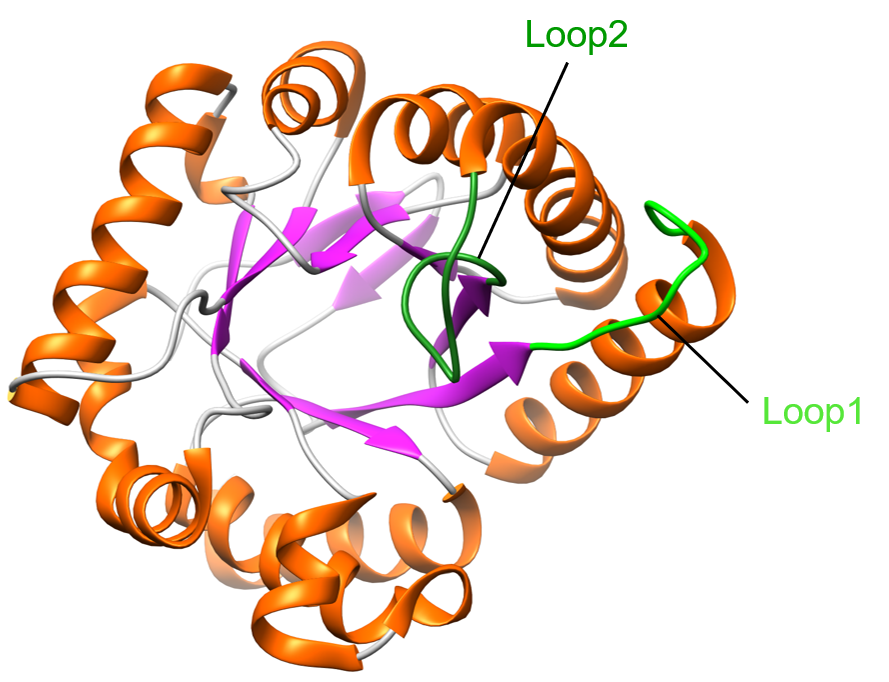
Two flexible loops in the structure adopt variable conformations (Figure 3) - loop1 (residues 27-37) and loop2 (residues 65-75). These loops are among the most conserved regions of DHPS. They are highly mobile and their mobility might be linked to DHPS catalysis (Levy et al., 2008). In the unliganded form of DHPS in B. anthracis, researchers identified a mechanism by which the pterin binding pocket is stabilized by loop2.
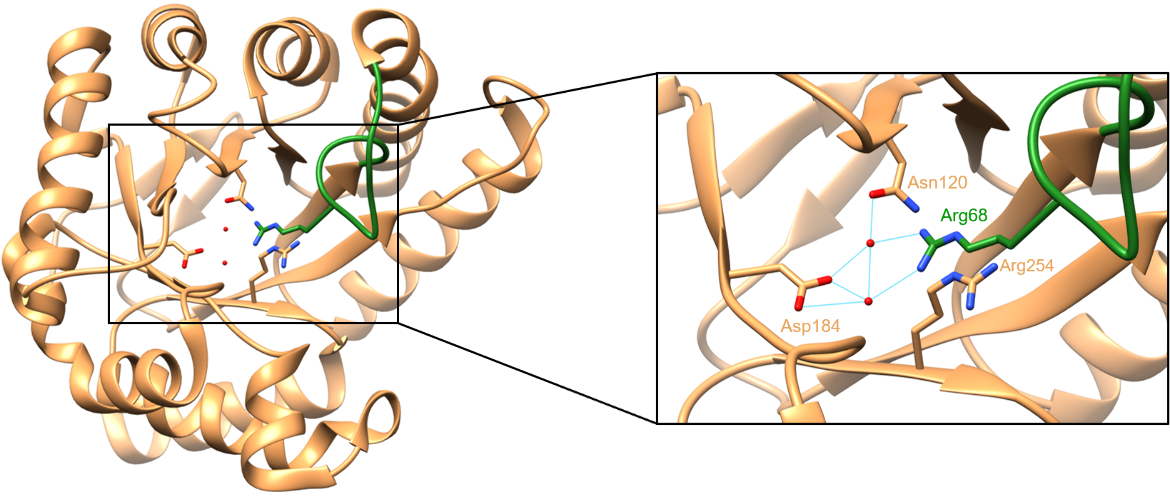
When no pterin substrate is bound, the side chain of Arg68 occupies the binding site. The guanidinium group of Arg68 interacts with two water molecules, resulting in a structure that resembles the structure of a pterin ring in terms of size and shape (Babaoglu et al., 2004). The enzyme appears to take advantage of this structural similarity. Since the pterin binding pocket creates a cavity in the core of the TIM barrel, its occupation by the guanidinium moiety helps stabilize the unliganded version of DHPS (Babaoglu et al., 2004). In the structure, Arg68 is stabilized by a π-stacking interaction with the side chain of Arg254 and water-mediated hydrogen bonds with Asn120 and Asp184. These interactions can be seen below in Figure 4.
|
| Figure 4. Interactions between Arg68, Asn120, Asp184, Arg254, and two water molecules. Hydrogen bonds are colored in cyan (PDB ID: 1tws, Babaoglu et al., 2004). |
Active Site
The pterin binding pocket is located in a cleft at the center of the TIM barrel, directly below the two flexible loops. A substrate analog of DHPS, 6-hydroxymethyl-pterine-pyrophosphate or PtPP is present in the structure examined here. This pterin substrate is primarily stabilized through direct hydrogen-bonding interactions (Figure 5). It forms hydrogen bonds with the side chains of Asn120, Asp184, and Lys220, as well as a conserved water molecule. There are two faces of the pterin ring in the substrate. One face stacks on the side chain of Arg254, while the other packs against Met145 and Phe189 (Babaoglu et al., 2004). All of these residues that interact with the pterin ring are conserved among bacterial DHPS enzymes. In the crystal structure below, loop2 is disordered due to the displacement of Arg68 caused by the binding of PtPP.
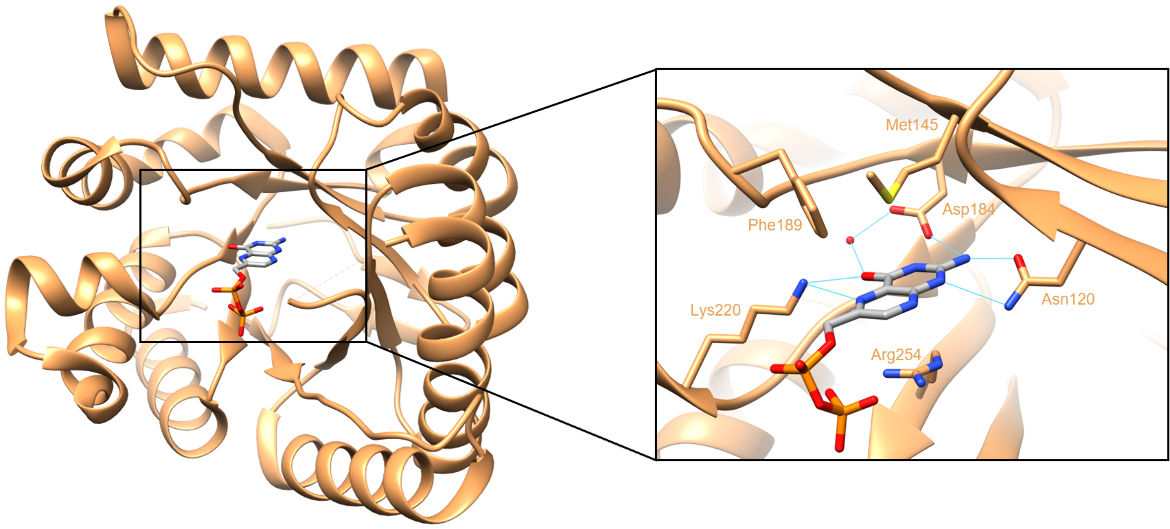
|
| Figure 5. Interactions between the DHPP analogue PtPP, and DHPS. Hydrogen bonds are colored in cyan (PDB ID: 1tww, Babaoglu et al., 2004). |
In the pABA binding site, the substrate is primarily stabilized through hydrogen bonding interactions between Ser221 of DHPS and the terminal carboxylate group of pABA. In addition to these hydrogen bonds, pABA is observed to sit against the acyl chain of Lys220 and make an edge-to-face π stacking interaction with Phe189 (Yun et al., 2012). The interactions between pABA and DHPS can be seen in Figure 6. In susceptible bacteria, sulfamethoxazole occupies the pABA binding pocket and prevents DHPS from binding pABA.
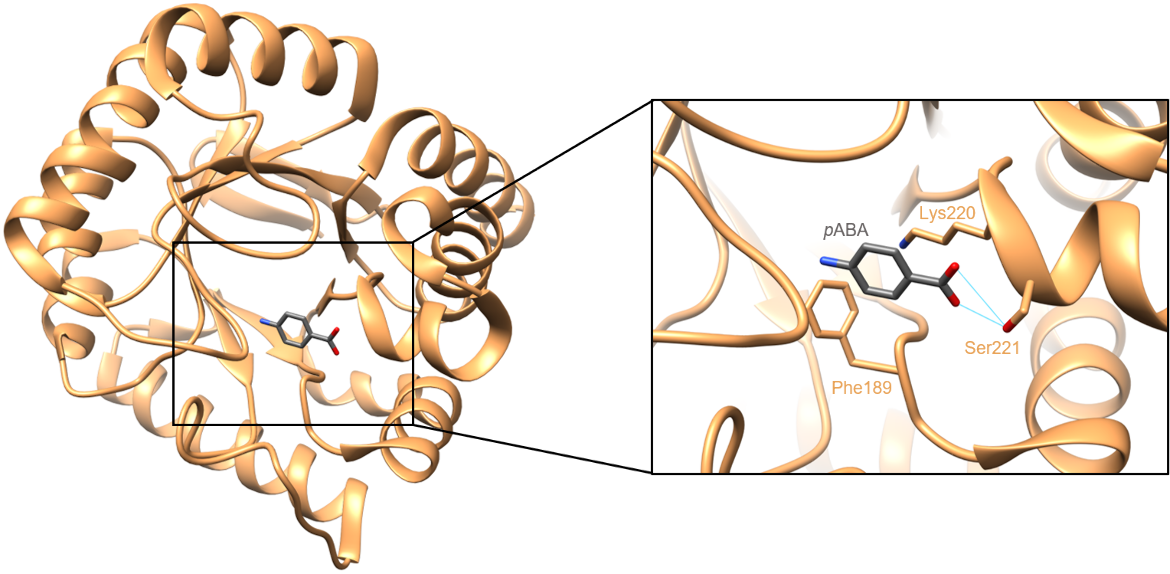
|
| Figure 6. Interactions between pABA and DHPS. Hydrogen bonds are colored in cyan (PDB ID: 3tyz, Yun et al., 2012). |
Synthesizing information from the structures of DHPS and its various substrate complexes has helped us understand the key steps in the catalytic mechanism of DHPS (Babaoglu et al., 2004):
* In the unliganded enzyme, the side chain of Arg68 from loop 2 occupies the pterin binding pocket at the center of the β-barrel. This conformation of the loop2 prevents the binding of pABA to the enzyme.
* The two DHPS substrates have an ordered binding to the enzyme, such that the conformational changes in the loops 1 and 2 regulate their binding. First dihydropteroate pyrophosphate (DHPP) binds to the enzyme (Vinnicombe and Derrick, 1999). This binding is stabilized by Asn27 and a Mg2+ ion that coordinates the pyrophosphate.
* This binding produces a conformational change in loop1 and loop2 (moving Arg68 out of the pterin binding pocket), making way for pABA binding.
* The nitrogen of pABA attacks the C9 position of DHPP to form dihydropteroate and pyrophosphate is lost. A conserved residue such as Asp35 is thought to play a key role in the catalysis.
* Once the pyrophosphate is released the enzyme reverts to its unliganded state and Arg68 reoccupies the pterin-binding pocket position described.
Pharmacological Implications
Folate synthesis begins with a series of steps that use a GTP molecule to prepare a precursor molecule. The DHPS enzyme catalyzes the joining of this precursor molecule with pABA to produce the first folate the tetrahydrofolate biosynthesis, and bacteria are dependent on its function (Yun et al., 2012). If DHPS is inhibited, bacteria can no longer produce dihydropteroate, a necessary precursor molecule in the tetrahydrofolate synthesis pathway. This would lead to cell death, as the bacteria would lack folate to use during DNA and protein synthesis. Thus the DHPS enzyme is an excellent antibiotic target.
References
Babaoglu, K., Qi, J., Lee, R., White, S. (2004). Crystal Structure of 7,8-Dihydropteroate Synthase from Bacillus anthracis. Structure, 12(9), 1705-1717. https://doi.org/10.1016/j.str.2004.07.011 PDB IDs: 1tws, 1tww
Banner, D., Bloomer, A., Petsko, G., Phillips, D., Pogson, C., Wilson, I., Corran, P., Furth, A., Milman, J., Offord, R., Priddle, J., Waley, S. (1975). Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5Å resolution: using amino acid sequence data. Nature, 255(5510), 609-614. https://doi.org/10.1038/255609a0
Fernández-Villa, D., Aguilar, M. R., Rojo, L. (2019). Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications. International Journal of Molecular Sciences, 20(20), 4996. https://doi.org/10.3390/ijms20204996
Levy, C., Minnis, D., Derrick, J. P. (2008) Dihydropteroate synthase from Streptococcus pneumoniae: structure, ligand recognition and mechanism of sulfonamide resistance. Biochemical Journal, 412, 379-388. doi: 10.1042/BJ20071598 https://hal.science/hal-00478924v1
Vinnicombe, H. G., Derrick, J. P. (1999) Dihydropteroate synthase from Streptococcus pneumoniae: characterization of substrate binding order and sulfonamide inhibition. Biochem Biophys Res Commun. 258(3):752-7. https://doi.org/10.1006/bbrc.1999.0695
Yun, M. K., Wu, Y., Li, Z., Zhao, Y., Waddell, M. B., Ferreira, A. M., Lee, R. E., Bashford, D., White, S. W. (2012). Catalysis and Sulfa Drug Resistance in Dihydropteroate Synthase. Science, 335(6072), 1110-1114. https://doi.org/10.1126/science.1214641 PDB ID: 3tyz
March 2025, Steven Arnold; Reviewed by Dr. Christina Bourne
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/folate-synth/DHS