RNA Polymerase
RNA polymerase (RNAP) is the enzyme that carries out transcription, the process of synthesizing molecules of ribonucleic acid (RNA) using DNA as a template and using ribonucleoside triphosphates (rNTPs) as substrates. Bacteria, archaea, and eukaryotes have structurally and mechanistically related RNA polymerases. While bacterial RNAP is comprised of five subunits, the archaeal, and eukaryotic enzymes have additional subunits. The differences in the polymer sequences of bacterial and eukaryotic RNAP have enabled the identification of inhibitors that can selectively target the bacterial enzyme. Currently, RNAP is the target of two classes of FDA-approved antibacterial agents, including some of the antibiotics listed here:
| rifampicin or rifampicin | rifapentine | rifabutin |
| rifamycin SV | rifaximin | fidaxomicin |
Function
The three major phases of transcription are initiation, elongation, and termination. In the initiation step, RNAP forms a holoenzyme and binds promoter DNA. In the elongation step, RNAP “reads” a template DNA strand and synthesizes a complementary molecule of RNA. In the termination step, the RNA transcript is released and RNAP prepares to re-enter initiation. Learn more about RNA synthesis.
Structure
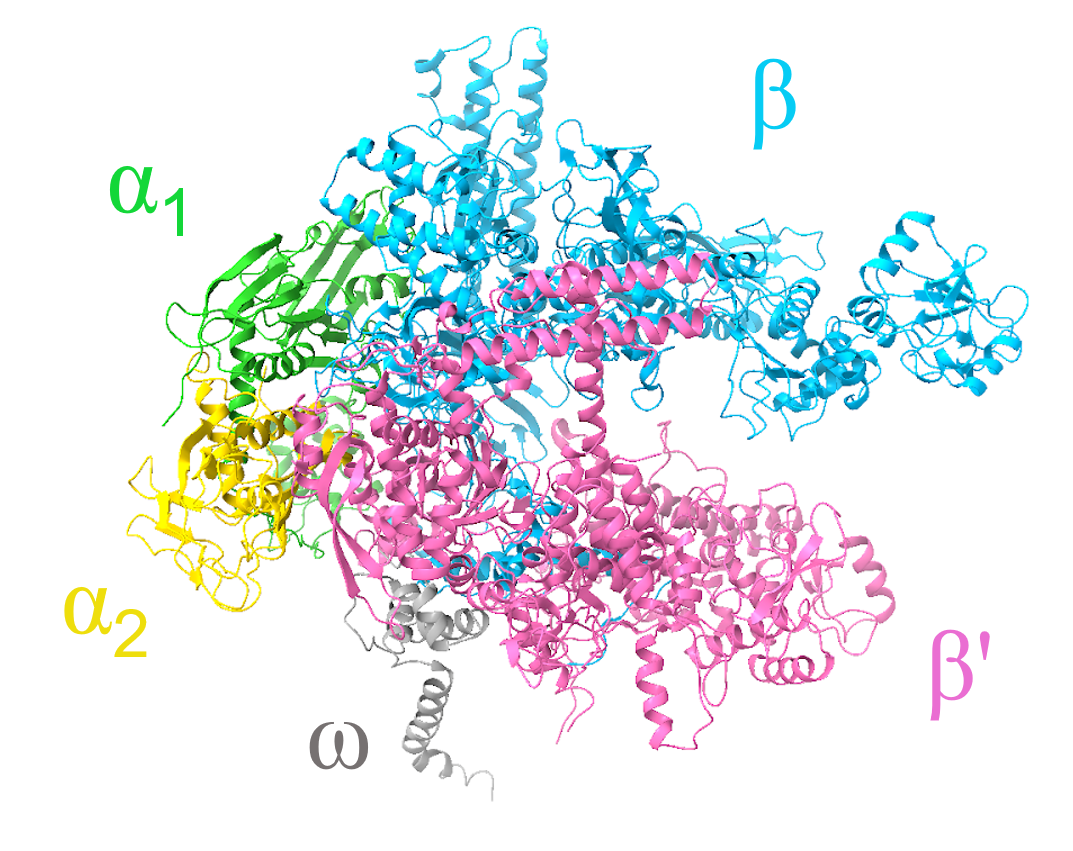
Bacterial RNAP is composed of five subunits (ɑ2ββ′ω) with a total molecular weight of ~380 kDa. The overall RNAP structure resembles a crab claw, with the β and β′ subunits representing the two pincers of the claw. The structure of Escherichia coli RNAP core enzyme (Murakami, 2013) shows that it consists of:
1. Two ɑ subunits, ɑ1 and ɑ2 (each 329 residues; colored green and yellow)
2. One β subunit (1342 residues; colored light blue)
3. One β′ subunit (1407 residues; colored pink)
4. One ω subunit (91 residues; colored dark gray)
|
| Figure 1. Structural overview of the Escherichia coli RNAP core enzyme shown in a ribbon representation (PDB ID: 4yg2, Murakami, 2013). |
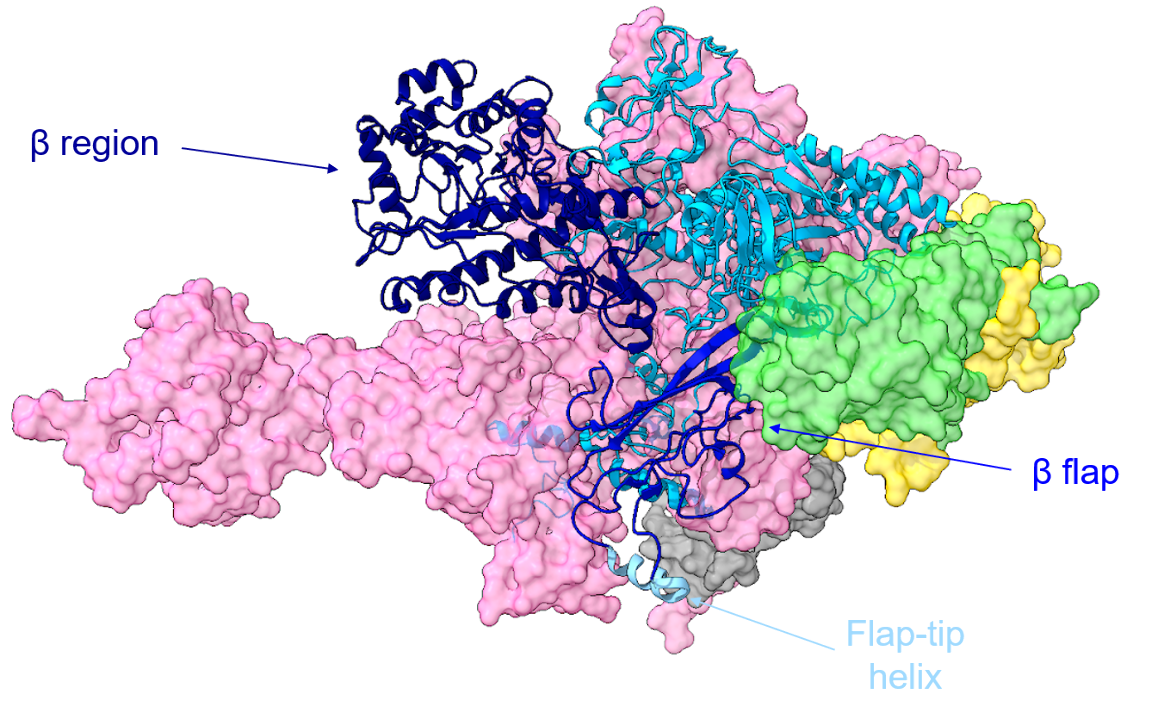
The structure of bacterial RNA polymerase holoenzyme from Thermus thermophilus has a similar structure (Vassylyev et al., 2002, Figure 2). It contains two identical ɑ subunits, shown in spacefilling representation and colored yellow and green. Each ɑ subunit protein contains an N-terminal domain (ɑNTD), a C-terminal domain (ɑCTD), and a long, flexible linker. The ɑNTD domains serve as a base for the RNAP assembly, while the ɑCTD makes interactions with DNA and transcription factors.
The β subunit contains part of the RNAP active center and plays a key role in the binding of DNA, RNA, and rNTPs. A ribbon model of the β subunit is shown in Figure 2, with different parts of the subunit colored in shades of blue.
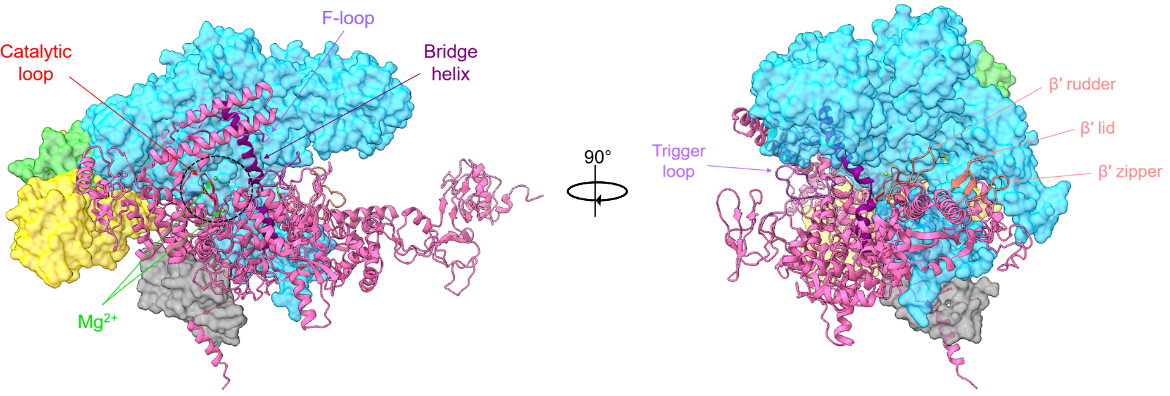
The β′ subunit is the largest subunit in RNAP. It contains the rest of the RNAP active center and is shown in cartoon representation in Figure 3. Key parts of this protein include three aspartate residues (D739, D741, and D743) that coordinate Mg2+ ions required for RNA synthesis; the bridge helix (E1041-T1066) that connects the β and β′ clamps; and the trigger loop (T1237-Q1254).
The interactions between the β and β′ subunits form a large internal cleft which creates three openings for molecules to enter and exit the active site region. The active-center cleft accommodates the RNA-DNA hybrid and downstream double-stranded DNA. The NTP-entrance channel (also referred to as the “secondary channel”) serves as the location for NTP entry. The “RNA-exit channel” serves as a place for the nascent RNA to exit RNAP. The switch region is located at the base of the β′ pincer (also referred to as the "clamp"), mediates the opening of RNAP active-center cleft to enable DNA entry in the early stages of transcription initiation, and closing of the RNAP active-center cleft to enable DNA retention in late stages of transcription initiation and transcription elongation.
The ⍵ subunit is the smallest subunit in bacterial RNAP (not shown in the figures above). It interacts mostly with the β′ subunit. The ⍵ subunit is not necessary for catalysis but assists assembly of RNAP and contributes to the stability of RNAP.
Active Site
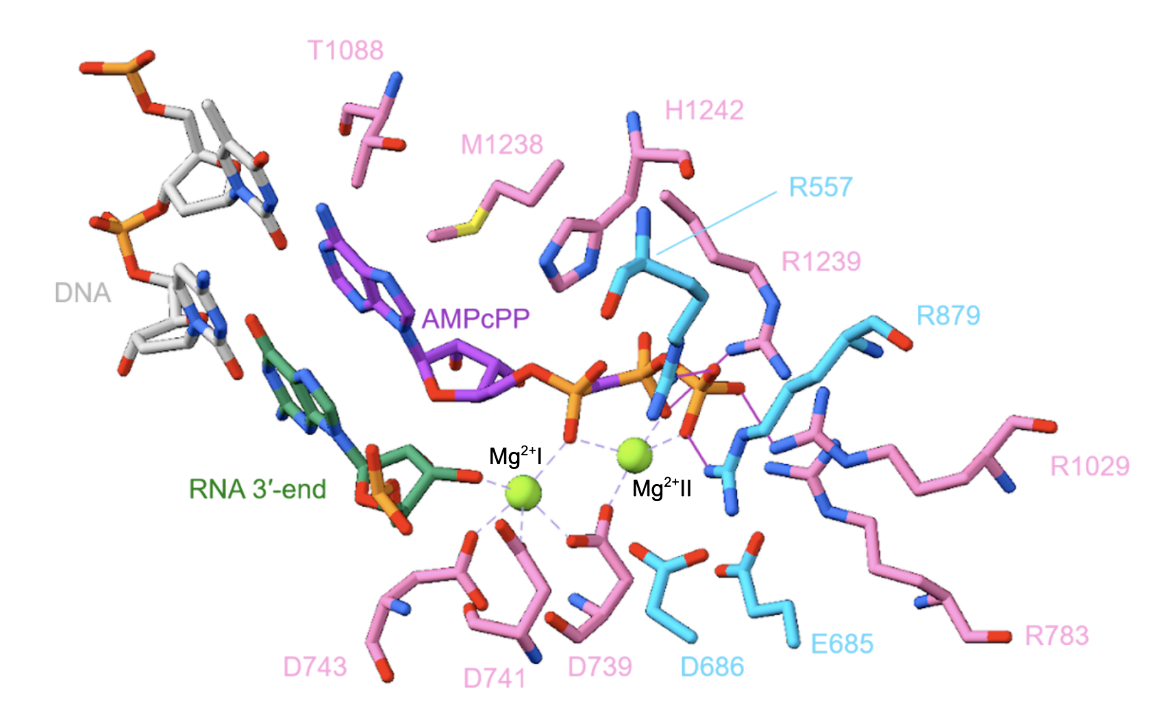
In each nucleotide-addition cycle, RNAP transfers the nucleotidyl moiety from an NTP to the terminal 3’-OH of a nascent RNA transcript and releases PPi.
Two Mg2+ ions play an important role in RNA synthesis. The Mg2+(I) ion interacts with the Asp residues of the β′ subunit (D739, D741, and D743) and is present on RNAP continuously (Figure 4, Vassylyev et al., 2007). The Mg2+(II) ion interacts with the three phosphates of the NTP, arrives with the NTP, and leaves with the PPi, at the end of each nucleotide-addition step. In each nucleotide-addition step, nucleophilic attack by the RNA 3'-OH on the NTP ɑ-phosphate occurs. The Mg2+ ions enhance the nucleophilicity of the RNAP 3’-OH, enhance the electrophilicity of the NTP ɑ-phosphate, and promote breakage of the bond between the ɑ- and β-phosphates.
Pharmacological Implications
Despite decades of research and development, the only clinically approved drugs that target RNAP are rifamycins (Campbell et al., 2001) and fidaxomicin (Boyaci et al., 2018, Lin et al., 2018). Rifamycins bind to a site adjacent to the RNAP active center and sterically block the extension of short RNA products into longer ones. Fidaxomicin binds to the RNA switch region and prevents the closing of the RNAP active-center cleft. Administration of these drugs leads to cell death in susceptible bacteria (Ho et al., 2009).
References
Boyaci, H., Chen, J., Lilic, M., Palka, M., Mooney, R. A., Landick, R., Darst, S. A., Campbell, E. A. (2018). Fidaxomicin jams Mycobacterium tuberculosis RNA polymerase motions needed for initiation via RbpA contacts. eLife, 7, e34823. https://doi.org/10.7554/elife.34823
Campbell, E. A., Korzheva, N., Mustaev, A., Murakami, K., Nair, S., Goldfarb, A., Darst, S. A. (2001) Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell. 104(6):901-12. https://doi.org/10.1016/s0092-8674(01)00286-0
Ho, M., Hudson, B., Das, K., Arnold, E., and Ebright, R. (2009) Structures of RNA polymerase-antibiotic complexes. Curr. Opin. Structl. Biol. 19, 715-723. https://doi.org/10.1016/j.sbi.2009.10.010
Lin, W., Das, K., Degen, D., Mazumder, A., Duchi, D., Wang, D., Ebright, Y.W., Ebright, R.Y., Sineva, E., Gigliotti, M., Srivastava, A., Mandal, S., Jiang, Y., Liu, Y., Yin, R., Zhang, Z., Eng, E.T., Thomas, D., Donadio, S., Zhang, H., Zhang, C., Kapanidis, A.N., Ebright, R.H. (2018) Structural Basis of Transcription Inhibition by Fidaxomicin (Lipiarmycin A3). Mol Cell. 70(1):60-71.e15. https://doi.org/10.1016/j.molcel.2018.02.026
Murakami, K. (2013). X-ray Crystal Structure of Escherichia coli RNA Polymerase σ70 Holoenzyme. Journal Of Biological Chemistry, 288(13), 9126-9134. https://doi.org/10.1074/jbc.m112.430900 PDB ID: 4yg2
Vassylyev, D., Sekine, S., Laptenko, O., Lee, J., Vassylyeva, M., Borukhov, S., Yokoyama, S. (2002). Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature, 417(6890), 712-719. https://doi.org/10.1038/nature752 PDB ID: 1iw7
Vassylyev, D., Vassylyeva, M., Zhang, J., Palangat, M., Artsimovitch, I., Landick, R. (2007). Structural basis for substrate loading in bacterial RNA polymerase. Nature, 448(7150), 163-168. https://doi.org/10.1038/nature05931 PDB ID: 2o5j
March 2025, Steven Arnold; Reviewed by Dr. Richard Ebright
10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/rna-synth/rp