RNA Synthesis
What is RNA?
Ribo Nucleic Acid (or RNA) is a biopolymer made up of nucleotides with ribose sugars attached to nitrogenous bases and phosphate groups (Figure 1). The nitrogenous bases include adenine, guanine, uracil, and cytosine. They are found in a broad range of organisms. In most living organisms it functions as an intermediary between the genetic blueprint molecule (DNA) and the effector molecules (proteins). In viruses, RNA molecules can even function as the genome. RNA can form specific three-dimensional shapes and function as enzymes.
|
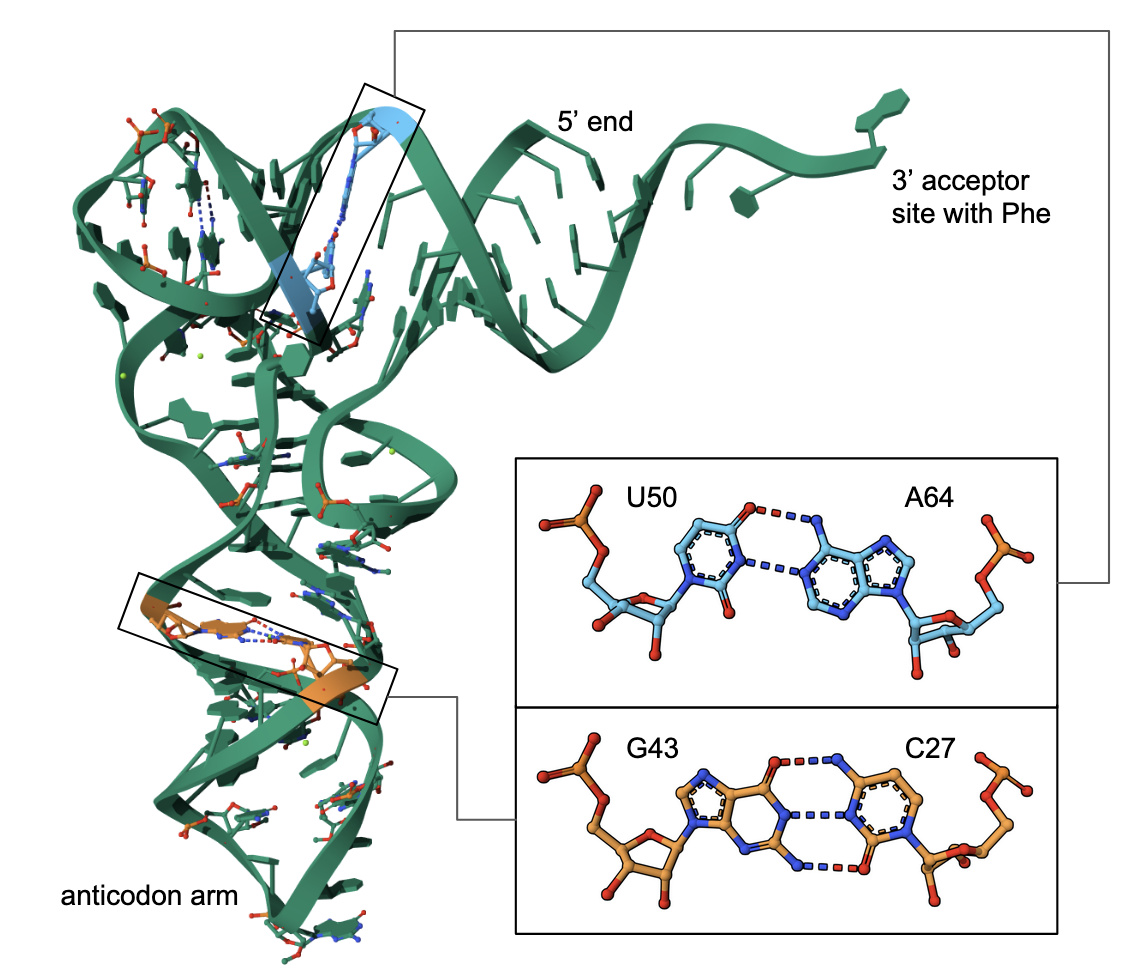
| Figure 1. The structure of yeast phenylalanine transfer RNA (PDB ID 1tra, Westhoff and Sundarlingam, 1986). Insets show closeups of different nucleotides that make up RNA. |
Structure of RNA
RNA mostly exists in the single-stranded form, but there are many RNA molecules seen in biology where all or parts of the structure may adopt a double helical structure. In fact, it is the ability of RNA molecules to base-pair that is used in the tRNA anti-codon loop's recognition of cognate codons in the mRNA. The genomes of some RNA viruses have double-stranded RNA. Learn more about the components and structure of RNA.
Types of RNA
Three main types of RNA are involved in protein synthesis (Wang and Farhana, 2023):
* messenger RNA (mRNA) - this type of RNA is transcribed from DNA and contains the genetic blueprint to make proteins. Since prokaryotic mRNA does not need to be processed, it can be translated into proteins immediately after they are made. Eukaryotic RNA undergoes maturation and splicing where non-coding regions (introns) are removed by a process called splicing and the coding regions (exons) are linked together. A protective cap (7-methylguanosine) is added to the 5’ end of the RNA transcript, and the 3’ end is polyadenylated for the stability of the mRNA.
* transfer RNA (tRNA) - this type of RNA helps translate mRNA into proteins. When considering their secondary structure - these molecules have a cloverleaf shape with a 3’ acceptor site, 5’ terminal phosphate, D arm, T arm, and anticodon arm. However, in its 3D folded state it forms an L-shaped molecule (see Figure 1).
* ribosomal RNA (rRNA) - this type of RNA forms ribosomes, where protein synthesis takes place. All ribosomes contain a large and small ribosomal subunit, with an exit (E), peptidyl (P), and acceptor (A) site to bind aminoacyl-tRNAs and link amino acids together to create polypeptides. In prokaryotic ribosomes, the small (30S) subunit contains a 16S RNA, while the large (50S) subunit contains 2 rRNA molecules (5S and 23S). Together these subunits make up a 70S ribosome. In eukaryotes, the small (40S) subunit contains an 18S rRNA, while the large (60S) subunit contains 3 rRNA molecules (5S, 5.8S, and 28S). Together these subunits form an 80S ribosome.
RNA Synthesis
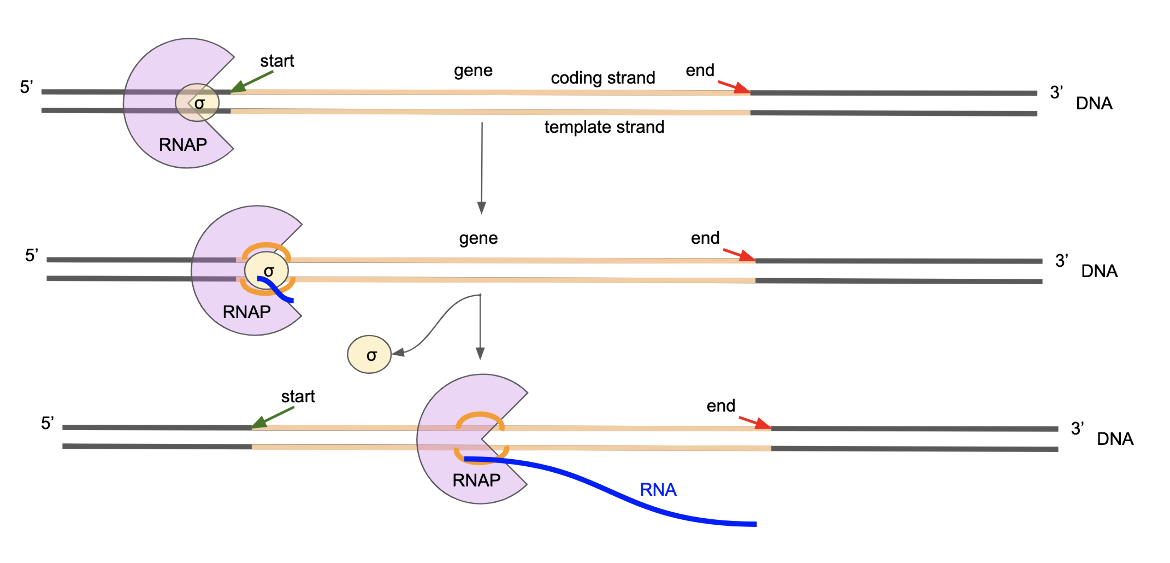
The process of transcription (making RNA based on a DNA template) is carried out by RNA polymerase (RNAP). In bacteria, this enzyme is responsible for all types of RNAs. It is composed of multiple protein subunits, called α, β, β′, and ω forming the core. Another factor σ also binds to this complex weakly to form the holoenzyme. Learn more about the structure of bacterial RNA polymerase.
Key steps in transcription include initiation, elongation, and termination (Winkelman et al., 2021). Key events in these steps are described here:
A. Initiation
* The RNAP core enzyme binds the transcription initiation factor σ (such as σ70 in E. coli) to yield the RNAP holoenzyme.
* The RNAP holoenzyme binds to a double-stranded promoter DNA in a sequence-specific manner, to form the RNAP-promoter closed promoter (RPc).
* The RNAP holoenzyme unwinds ~13 base pairs of DNA, forming an unwound "transcription bubble" to form the RNAP promoter open complex (RPo). Here the genetic information in the DNA template strand is accessible for transcription.
* rNTPs enter the holoenzyme, causing the RPo to convert to an initial transcribing complex (RPinit). The first phosphodiester bonds between rNTPs are formed as the enzyme begins to synthesize RNA.
* The RNAP holoenzyme synthesizes the first ~10 nucleotides of RNA as an RNAP-promoter initial transcribing complex (RPitc). In this step the RNAP remains stationary on promoter DNA, unwinds downstream DNA, and pulls the unwound downstream DNA into itself and past its active center, using a "scrunching" mechanism. Accommodating the additional unwound DNA in the transcription bubble introduces bulges and accumulates stress energy.
* After synthesis of ~11 nucleotides of RNA using the scrunching mechanism, the RNAP accumulates enough stress energy to break the sequence-specific interactions with promoter DNA. The accumulated stress energy also helps rewind the upstream of the transcription bubble, and RNAP escapes the promoter to form the transcription elongation complex (TEC).
B. Elongation
* The TEC uses a "stepping" mechanism where the RNAP translocates forward by 1 base pair for each nucleotide added to the nascent RNA. At this stage, the transcription initiation factor σ can be released during promoter escape or retained and released during subsequent steps in transcription elongation.
* The length of the transcription bubble length is maintained at ~10 base pairs during transcription elongation. The ~9-10 nucleotides at the 3' end of the nascent RNA (i.e., the most recently synthesized ~9-10 nucleotides) base-pair with the DNA template strand to form an RNA-DNA hybrid.
* During processive transcription elongation, the nucleotide addition proceeds at a rate of 1 nucleotide per ~30 ms.
* The rate of RNAP transcription during elongation can be, and frequently is, interrupted by specific DNA sequences or factor-dependent pauses. The factors include DNA-binding proteins, RNAP-binding transcription factors, and small-molecule effectors.
C. Termination
* When RNAP encounters an intrinsic terminator or a factor-dependent terminator RNAP stops moving, stops synthesizing RNA, releases the RNA product, and dissociates from DNA. Intrinsic terminators are DNA sequences that, when transcribed, yield a G/C-rich RNA hairpin followed by a U/A-rich RNA-DNA hybrid. On the other hand, a factor-dependent terminator is a DNA sequence that, when transcribed, yields a C-rich RNA sequence and is specifically recognized by the transcription termination factor Rho.
* Upon termination the RNAP releases the RNA transcript and dissociates from the RNA template. RNAP is then able to bind to a σ factor and re-enter initiation.
RNA Synthesis as a Target for Antibiotics
Transcription is the first step of gene expression and protein synthesis. It is necessary for cell survival and growth. Thus the RNA polymerase (RNAP) enzyme and its interactions with the various transcription factors present excellent targets for broad-spectrum antibacterial action (Ho et al., 2009; Lin et al., 2018; and Boyachi et al., 2018). Moreover, the bacterial RNAP and its functions are significantly different from those of its eukaryotic counterpart, so inhibiting it is likely to impact host cell functions. Currently, two classes of antibacterials that target RNAP are approved for use by the US FDA - rifamycin series and the lipiarmycin antibiotics.
References
Boyaci, H., Chen, J., Lilic, M., Palka, M., Mooney, R. A., Landick, R., Darst, S. A., Campbell, E. A. (2018). Fidaxomicin jams Mycobacterium tuberculosis RNA polymerase motions needed for initiation via RbpA contacts. eLife, 7, e34823. https://doi.org/10.7554/elife.34823
Ho, M., Hudson, B., Das, K., Arnold, E., and Ebright, R. (2009) Structures of RNA polymerase-antibiotic complexes. Curr. Opin. Structl. Biol. 19, 715-723. https://doi.org/10.1016/j.sbi.2009.10.010
Lin, W., Das, K., Degen, D., Mazumder, A., Duchi, D., Wang, D., Ebright, Y.W., Ebright, R.Y., Sineva, E., Gigliotti, M., Srivastava, A., Mandal, S., Jiang, Y., Liu, Y., Yin, R., Zhang, Z., Eng, E.T., Thomas, D., Donadio, S., Zhang, H., Zhang, C., Kapanidis, A.N., Ebright, R.H. (2018) Structural Basis of Transcription Inhibition by Fidaxomicin (Lipiarmycin A3). Mol Cell. 70(1):60-71.e15. https://doi.org/10.1016/j.molcel.2018.02.026
Wang, D., Farhana, A. (2023) Biochemistry, RNA Structure. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK558999/
Westhof, E., Sundaralingam, M. (1986) Restrained refinement of the monoclinic form of yeast phenylalanine transfer RNA. Temperature factors and dynamics, coordinated waters, and base-pair propeller twist angles. Biochemistry. 25(17):4868-78. https://doi.org/10.1021/bi00365a022
Winkelman, J., Nickels, B., and Ebright, R. H. (2021) The transition from transcription initiation to transcription elongation: start-site selection, initial transcription, and promoter escape. In RNA Polymerases as Molecular Motors, Second Edition. eds. Landick, R., Wang, J., and Strick, T. (RSC Publishing, Cambridge, UK), pp. 1-24. https://doi.org/10.1039/9781839160561-00001
March 2025, Steven Arnold, Shuchismita Dutta; Reviewed by: Dr. Richard Ebright
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/rna-synth