Cefoxitin Resistance
Susceptibility Testing
When possible, antibacterial substances, such as cefoxitin, are tested for their effectiveness against various infectious pathogens. These test results allow clinicians to choose the antibiotic likely to result in the most effective treatment of a particular bacterial infection. For instance, one such susceptibility test provides minimum inhibitory concentration (MIC) values that can then be used to identify a pathogenic bacterial strain as susceptible, intermediate, or resistant to a certain antibiotic.
A lower MIC value indicates that a lower concentration of the antibiotic is needed to inhibit the growth of the bacterial pathogen, meaning that the microorganism is susceptible to the drug. Therefore, using antibiotics with lower MIC values would result in more effective treatment of an infection. As can be seen in Table 6, it takes a relatively low concentration of cefoxitin to inhibit susceptible strains, while it would take a much greater concentration of the drug to restrict the growth of the resistant strains.
Table 6. Minimum inhibitory concentrations (MIC) that would classify the pathogenic bacterial strain as susceptible to cefoxitin, intermediate, or resistant to cefoxitin. Based on a dosing regimen of 2g every 6 hours. Adapted from (FDA, 2017). These values may not be the latest approved by the US FDA.
| Pathogen | MIC (µg/mL) for Susceptible (S) strains | MIC (µg/mL) for Intermediate (I) strains | MIC (µg/mL) Resistant (R) strains |
|---|---|---|---|
| Enterobacteriaceae | ≤4 | 8 | ≥16 |
| Neisseria gonorrhoeae | ≤2 | 4 | ≥8 |
| anaerobic bacteria | ≤4 | 8 | ≥16 |
Resistance Mechanism(s)
Cefoxitin resistance occurs when the antibiotic is not able to treat the infections it is intended to because the bacterial strains causing these infections have developed mechanisms to prevent the drug from functioning. These mechanisms include (CARD, 2017):
* Reduced permeability to antibiotic
* Antibiotic target replacement
* Antibiotic inactivation
Reduced Permeability to Antibiotic
β-lactam antibiotics, including cefoxitin, permeate through the outer membrane of gram-negative bacteria and reach their intracellular PBP targets using outer membrane proteins (OMPs), known as porins. However, if the bacterial cell reduces the expression of porins or expresses mutated porins so that it is no longer able to translocate the β-lactam through the membrane, the antibiotic will no longer be able to enter the cell, inhibit PBP activity, and kill the cell. As a result, the bacterium becomes less susceptible to the antibacterial agent. Some examples of resistance involving bacterial porins are described below in Table 7 (CARD, 2017).
Learn more about porins.
Table 7. The effect of decreased bacterial cell permeability on cefoxitin susceptibility. The causes of resistance were identified using CARD.
| Cause of Resistance | Description |
|---|---|
| Omp1 | Omp1 is an outer membrane porin in the bacteria Serratia marcescens. When this porin is absent due to a mutation in or deletion of the omp1 gene, cefoxitin is no longer able to enter the bacterial cell and bind to its target protein. This results in resistance to cefoxitin. |
| OmpK37 | OmpK37 is an outer membrane porin found in Klebsiella pneumoniae. This porin has a narrower channel compared to the other porins found in this bacteria (OmpK35 or OmpK36), which restricts the passage of cefoxitin into the cell. Strains expressing OmpK37 are less susceptible to cefoxitin. |
| Omp38 | When Burkholderia pseudomallei was expressed in Omp-deficient Escherichia coli cells, it was found that the permeability of cefoxitin in the cells decreased, thus reducing their susceptibility. |
| OmpK35 | OmpK35 is an outer membrane porin protein found in Klebsiella pneumoniae. In β-lactam-resistant Klebsiella, this porin is often deleted, limiting the diffusion of the antibiotic into the cell. |
Antibiotic Target Replacement
Methicillin-resistant Staphylococcus aureus (MRSA) strains that express the PBP2a protein will also be resistant to cefoxitin (Paterson et al., 2014). Resistance develops because PBP2a has a lower binding affinity for β-lactams, like cefoxitin; therefore, even in the presence of the antibiotic, the enzyme can still function normally and cross-link peptidoglycan strands, producing a rigid cell wall.
Learn more about PBP2a.
Antibiotic Inactivation
When a β-lactam antibiotic, like cefoxitin, enters the bacterial cell, in addition to its intended PBP target it may also bind to β-lactamases. These enzymes inactivate the drug by breaking the amide bond, which causes the functional β-lactam ring to open up. As a result, the antibiotic is no longer able to inhibit its target PBP enzyme.
Learn more about breaking bonds in β-lactam antibiotics.
In general, cefoxitin is a poor substrate for many β-lactamases (Matsuhashi and Tamaki, 1978), due to its 7α methoxy group, which is a characteristic of cephamycin antibiotics (CARD). Many infections resistant to penicillin antibiotics, such as penicillin G and ampicillin, respond to cefoxitin treatment (FDA, 2017). Although cefoxitin is stable against many of these enzymes, there are a variety of β-lactamases that are still able to destroy the antibiotic and confer resistance to cefoxitin, (as identified by CARD).
Table 8. A subset of β-lactamases that confer resistance to cefoxitin (as identified by CARD).
| Cause of Resistance | Description |
|---|---|
| CfxA | Class A β-lactamase found in Bacteroides vulgatus. |
| NmcA | Class A serine β-lactamase identified in Enterobacter cloacae. |
| CAM-1 | Central Alberta Metallo (CAM) β-lactamase identified in strains of Pseudomonas aeruginosa making them resistant to broad-spectrum antibiotics. |
| NDM-5 | A New Delhi metallo-enzyme identified in Escherichia coli. It is a class B β-lactamase. |
| CMY-2 | Class C β-lactamase found in Escherichia coli |
| blaC | a broad-spectrum class A β-lactamase encoded in the chromosome of Mycobacterium tuberculosis. |
| blaS1 | a β-lactamase found in Mycolicibacterium smegmatis |
| CTX-M-3 | a β-lactamase found in Citrobacter freundii |
| PDC-3 | an extended-spectrum β-lactamase found in Pseudomonas aeruginosa |
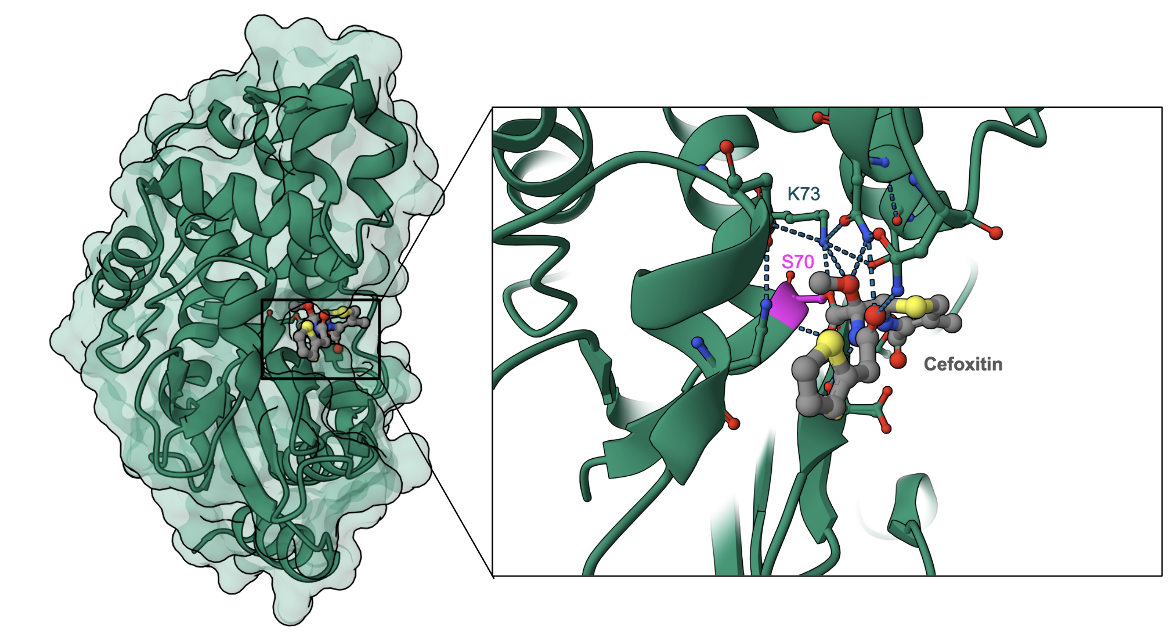
Here we show the covalent binding of cefoxitin to a CTX-M-9 β-lactamase from Escherichia coli (Figure 6). Note that the antibiotic's β-lactam ring is opened and forms a covalent bond with Ser70 of the enzyme (Chen et al., 2005). Besides the direct hydrogen bonds between Lys73, several other amino acid residues (Asn104, Asn132, Ser130, and Thr235) form non-covalent bonds with the antibiotic.
Back to the article on cefoxitin.
References
Chen, Y., Shoichet, B., Bonnet, R. (2005) Structure, function, and inhibition along the reaction coordinate of CTX-M beta-lactamases. J Am Chem Soc. 127, 5423-34. https://doi.org/10.1021/ja042850a
Matsuhashi, M., Tamaki, S. (1978) Enzymatic studies on the mechanism of action of cefoxitin. Correlation between the affinities of cefoxitin to penicillin-binding proteins and its rates of inhibition of the respective penicillin-sensitive reactions in E. coli. J Antibiot (Tokyo). 31, 1292-5. https://doi.org/10.7164/antibiotics.31.1292
Paterson, G. K., Harrison, E. M., and Holmes, M. A. (2014). The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends in Microbiology, 22, 42-47. https://doi.org/10.1016/j.tim.2013.11.003