Sulfonamide
Discovery
In 1927, Bayer, a subsidiary of the german dye industry syndicate (I. G. Farbenindustrie) began screening for dyes with antibacterial effects in animals. As a part of this effort, Dr. Gerhard Domagh and his colleagues created a sulfonamide-containing dye called Prontosil rubrum (Shambaugh, 1966). It was tested on mice in 1931 and found to be effective against streptococcal infections. The drug was successfully tested on a human subject in 1933 when a 10-year-old boy dying of staphylococcal septicemia was treated with this drug and recovered. It was deemed non-toxic in small doses. A few years later, in 1937, it was shown that the sulfanilamide part of Prontosil rubrum was active as an antibiotic (van Miert, 1994). In 1939 Gerhard Domagk was awarded the Nobel Prize in Physiology or Medicine for the discovery of the antibacterial effects of prontosil. Although Dr. Domagk was prevented from accepting the prize at the time by political conditions, in 1947 he received the gold medal and the diploma (Physiology or Medicine 1939).
In the 1940s, a large number of sulfonamide compounds were synthesized to find drugs with better antibacterial activity, solubility, absorption, and reduced toxicity for use in animals. In the late 1950s, a number of sulfonamides e.g., sulfadimethoxine, sulfamethoxypyridazine, and sulfamethoxazole were developed as safer, lesser toxic molecules for more prolonged use (van Miert, 1994). Research on the side effects of sulfonamides resulted in the development of diuretics (e.g., Acetazolamide) and antidiabetogenic agents (e.g., sulfonylureas) (van Miert, 1994).
Overview of Chemistry
Sulfonamides are organosulfur compounds containing the -SO2NH2 and/or -SO2NH- as the main functional group (Figure 1) and characteristic 6- or 5-membered heterocyclic rings (Ovung and Bhattacharya, 2021). These molecules are not readily biodegradable and can have various side effects impacting the digestive and respiratory tracts. Some individuals have developed severe allergies to this class of drugs (often called sulfa-drugs), commonly manifested as rashes. Studies have revealed that the additional groups in a sulfonamide molecule, and not the sulfonamide functional group, may be responsible for the allergies (Giles et al., 2019).
Antimicrobial sulfonamides contain an arylamine group and a five- (or six-) membered, nitrogen-containing ring on the opposite end of the sulfonamide functional group (Figure 1A). The arylamine group mimics the structure of para-aminobenzoic acid (pABA, Figure 1B), a substrate for the enzyme in the folate biosynthetic pathway that this class of drug inhibits. In fact, research on sulfonamides led to the discovery of pABA (Woods 1940). Thus the arylamine group is a required feature in all antimicrobial sulfonamides (Giles et al., 2019). The 5-membered heterocyclic ring makes the molecule more acidic and increases its solubility, also playing a role in sulfonamides' antimicrobial function. Learn more about folate synthesis.
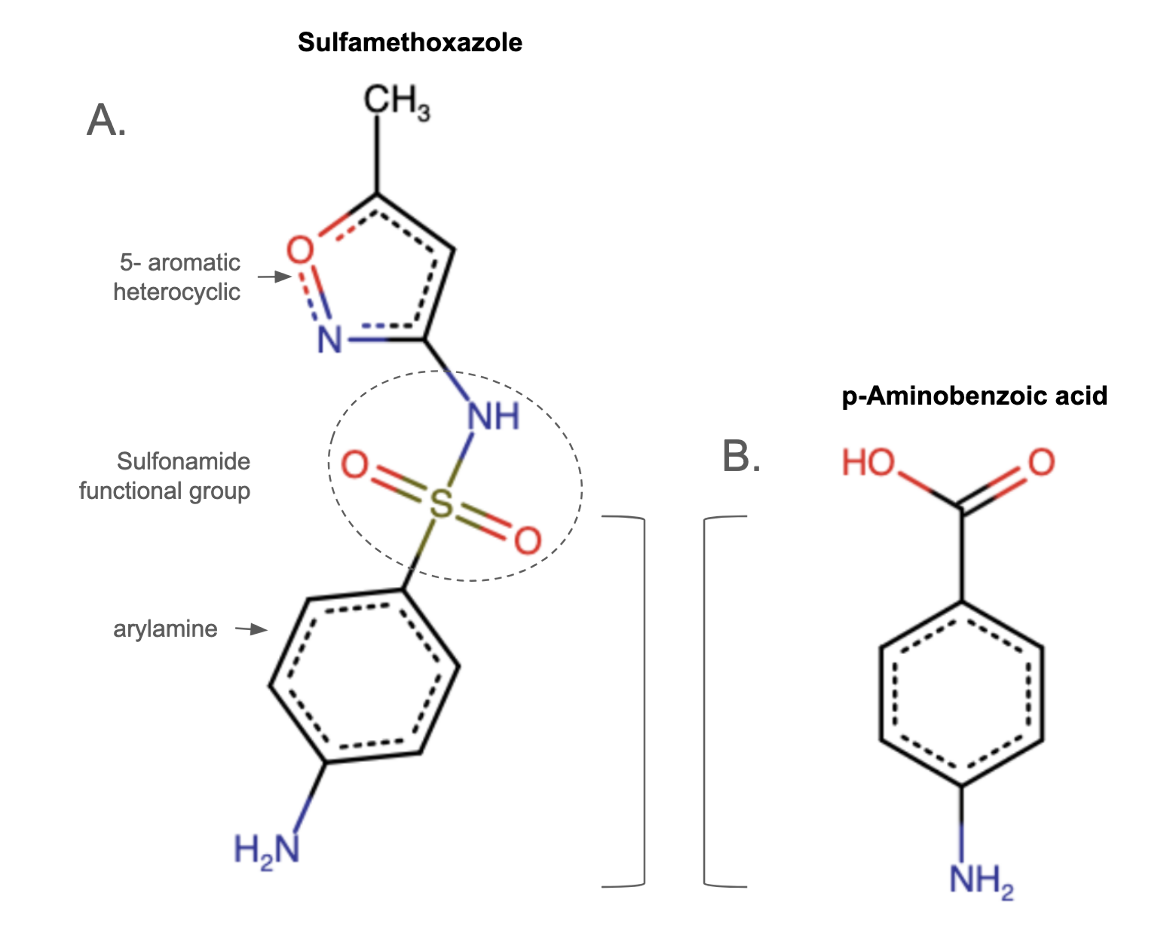
|
| Figure 1. 2D chemical drawings of a sulfonamide and the molecule it mimics A. Sulfamethoxazole (CC ID 08D) showing the key chemical groups. B. 4-aminobenzoic acid or para-aminobenzoic acid (pABA). |
Types
Sulfonamides were developed initially as antimicrobials but over time have yielded many other classes of drugs. For this discussion (and also for studying various sulfonamide allergies) this class of molecules can be divided into two groups (Ovung and Bhattacharya, 2021):
(i) antibacterial sulfonamides: these molecules include an aromatic amine
(ii) non-antibacterial sulfonamides: these molecules are without an aromatic amine.
The antimicrobials include molecules such as Sulfamethoxazole, while non-antibacterial sulfonamides include antivirals, carbonic anhydrase inhibitors, cyclooxygenase-2- selective nonsteroidal antiinflammatory drugs, loop and thiazide diuretics, and sulfonylureas. Since the latter group does not include both the N-containing ring and arylamine groups current research does not clinically justify any cross-reactivity between allergy to an antibiotic sulfonamide and the use of a non-antibacterial sulfonamide drug (Wulf and Matuszewski, 2013).
Learn more about sulfa drug allergies.
Resistance
Since sulfonamides are frequently used antibiotics for animals (e.g. cattle) and humans, a high fraction of these drugs, excreted without metabolism, are released into the environment as manure or sewage (Ovung and Bhattacharya, 2021). Accumulation of these antibiotics within the soil can impact soil microbial communities, where sulfonamide-resistant bacteria with resistance genes (e.g., sul1, sul2, sul3) have been identified (Wang et al., 2014). Learn more about Sulfamethoxazole Resistance.
References
Giles, A., Foushee, J., Lantz, E., Gumina, G. (2019) Sulfonamide Allergies. Pharmacy (Basel). 7(3):132. https://doi.org/10.3390/pharmacy7030132
Ovung, A., Bhattacharyya, J. (2021) Sulfonamide drugs: structure, antibacterial property, toxicity, and biophysical interactions. Biophys Rev. 13(2):259-272. https://doi.org/10.1007/s12551-021-00795-9
Physiology or Medicine 1939 – Presentation Speech. NobelPrize.org. Nobel Prize Outreach 2025. Tue. 28 Jan 2025.
Shambaugh G. E. (1966) History of Sulfonamides. Arch Otolaryngol. 83(1):1–2. doi:10.1001/archotol.1966.00760020003001
van Miert AS. (1994) The sulfonamide-diaminopyrimidine story. J Vet Pharmacol Ther. 17(4):309-16. https://doi.org/10.1111/j.1365-2885.1994.tb00251.x
Wang, N., Yang, X., Jiao, S., Zhang, J., Ye, B., Gao, S. (2014) Sulfonamide-resistant bacteria and their resistance genes in soils fertilized with manures from Jiangsu Province, Southeastern China. PLoS One. 9(11):e112626. https://doi.org/10.1371/journal.pone.0112626
Woods, D. D. (1940) The Relation of p-aminobenzoic Acid to the Mechanism of the Action of Sulphanilamide. Br J Exp Pathol. 21(2):74–90. PMCID: PMC2065323.
Wulf, N.R., Matuszewski, K.A. (2013) Sulfonamide cross-reactivity: is there evidence to support broad cross-allergenicity? Am J Health Syst Pharm. 70(17):1483-94. https://doi.org/10.2146/ajhp120291
March 2025, Shuchismita Dutta; Reviewed by Dr. Christina Bourne
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/folate-synth/DHS/sulfonamide



