Plazomicin
Drug Name
Plazomicin is a relatively new or next-generation aminoglycoside, that is used to treat drug-resistant bacteria, including carbapenem-resistant (CRE) and extended-spectrum β-lactamase (ESBL) producing Enterobacteriaceae. It is administered intravenously to treat complicated urinary tract infections and other infections that have limited or no alternative treatment options.
Table 1. Basic profile of plazomicin.
| Description | Synthetically derived next-generation aminoglycoside |
| Target(s) | Ribosomal 30S subunit |
| Generic | Plazomicin |
| Commercial Name | Zemdri |
| Combination Drug(s) | N/A |
| Other Synonyms | N/A |
| IUPAC Name | (2S)-4-amino-N-[(1R,2S,3S,4R,5S)-5-amino-4-{[(2S,3R)-3-amino-6-{[(2-hydroxyethyl)amino]methyl}-3,4-dihydro-2H-pyran-2-yl]oxy}-2-{[(2R,3R,4R,5R)-3,5-dihydroxy-5-methyl-4-(methylamino)oxan-2-yl]oxy}-3-hydroxycyclohexyl]-2-hydroxybutanamide |
| Ligand Code in PDB | EDS |
| PDB Structure | 7lh5 |
| ATC code | J01GB14 |
|
|
|
Antibiotic Chemistry
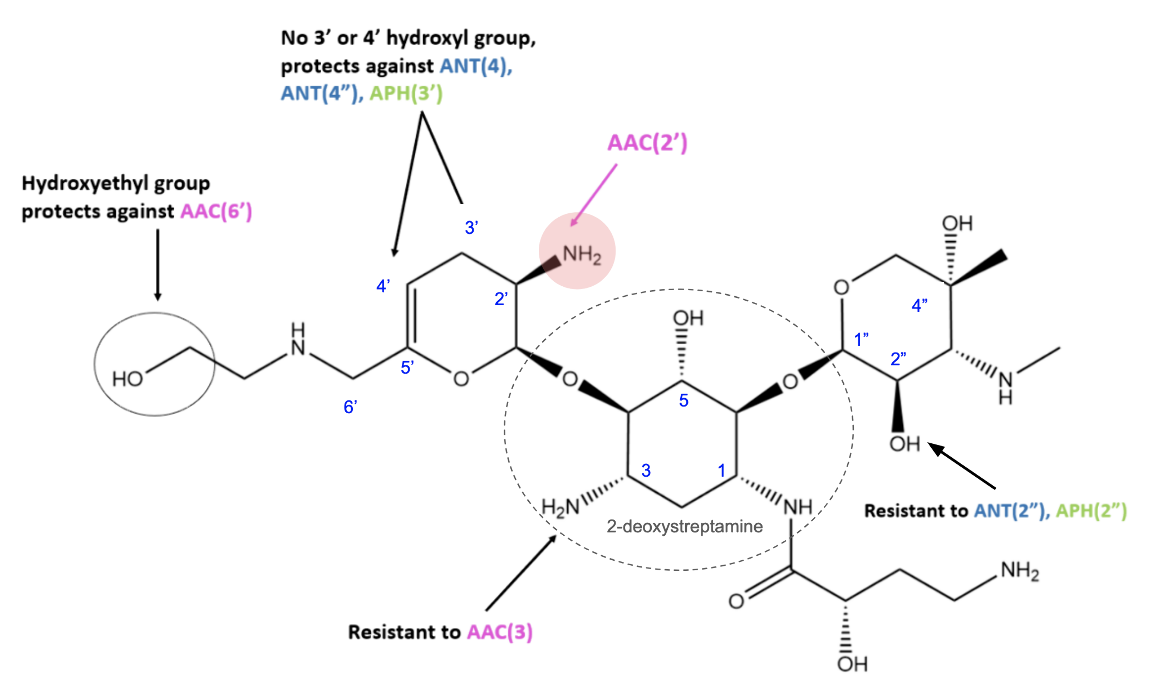
Plazomicin was synthesized from a sisomicin and designed to be resistant to the action of a variety of aminoglycoside modifying enzymes (AMEs) commonly found in gram-negative pathogens (Krause et al., 2016). It has the 2-deoxystreptamine ring in its core but the substitutions around the ring are different so that it can not be modified by APH(3')-III, -VI, and -VII phosphotransferases and ANT adenyltransferase (see Figure 2). Modification at the N-1 position sterically hinders the action of the AAC(3) acetyltransferases, ANT(2"), and APH(2") enzymes, while the addition of a hydroxyethyl substituent at the 6' position inhibits the action of the AAC(6') enzymes (Golkar et al., 2021). Plazomicin also lacks hydroxyl groups at the 3′ and 4′ positions, protecting it against the activity of AMEs ANT(4′) and APH(3′).
Learn more about AMEs that act on plazomicin.
Learn about other aminoglycoside modifying enzymes.
Drug Information
Table 2. Chemical and physical properties (DrugBank).
| Chemical Formula | C25H48N6O10 |
| Molecular Weight | 592.691 g/mol |
| Calculated Predicted Partition Coefficient: cLogP | -2.2 |
| Calculated Predicted Aqueous Solubility: cLogS | -1.7 |
| Solubility (in water) | 12.3 mg/mL |
| Predicted Topological Polar Surface Area (TPSA) | 269.29 Å2 |
Drug Target
The ribosome is the macromolecular machine on which proteins are synthesized. It is targeted by many classes of antibiotics approved by the US FDA. Plazomicin positions itself in the decoding center of the 30S ribosomal subunit and makes direct contact with rRNA. It inhibits the accommodation of tRNAs in the A-site, as a result, incorrect amino acids are inserted into nascent polypeptide chains during translation, producing nonfunctional proteins.
Learn more about mRNA decoding, protein synthesis, and ribosomes.
Drug-Target Complex
The structure of Thermus thermophilus ribosome in complex with plazomicin reveals that the antibiotic binds to the 16S ribosomal A site. It interacts vi hydrogen bonds with G1405, C1407, A1408, G1494, U1495, C1496, and G1497 (Figure 3). These interactions in the aminoacyl-tRNA site (A-site) likely interfere with the fidelity of mRNA translation (Golkar et al., 2021).
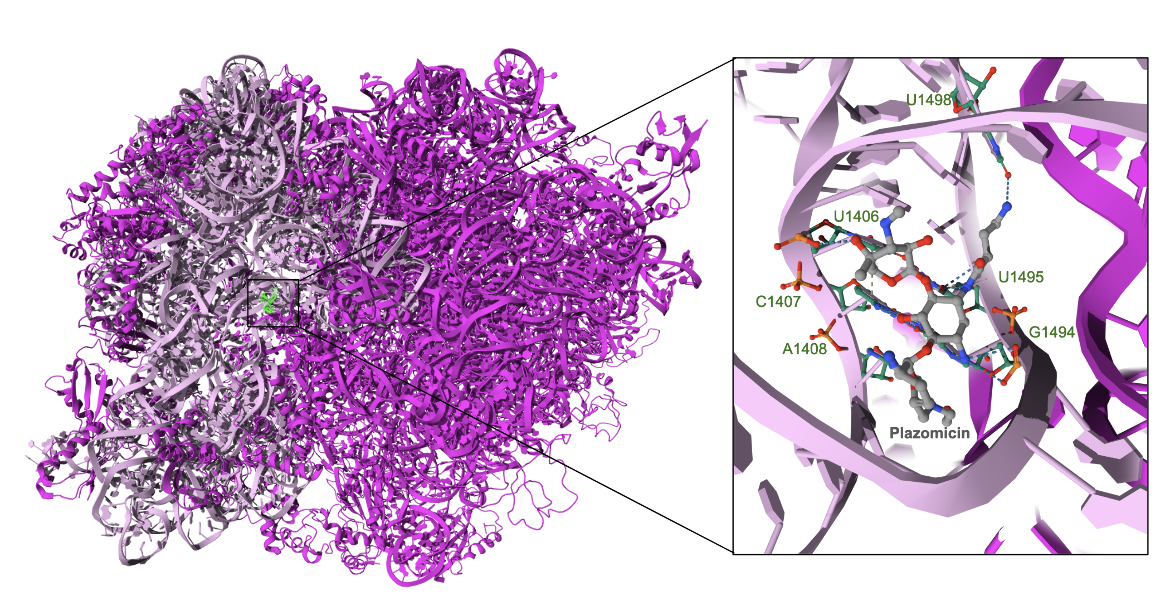
|
| Figure 3. The binding site of plazomicin and the major interactions of the drug with the ribosome (PDB ID: 7lh5, Golkar et al., 2021). |
Pharmacologic Properties and Safety
Table 3. Pharmacokinetics: ADMET of plazomicin.
| Features | Comment(s) | Source |
|---|---|---|
| Oral Bioavailability (%) | 0 | DrugBank |
| IC50 | N/A | N/A |
| Ki (μM) | N/A | N/A |
| Half-Life (hrs) | 3.5 ± 0.5 hours | DrugBank |
| Duration of Action | 24 hours | FDA |
| Absorption Site | Rapidly absorbed into the extracellular components of body tissues after injection | DrugBank |
| Transporter(s) | Multidrug and toxin extrusion protein 1 (MATE1) and MATE2 | DrugBank |
| Metabolism | Plazomicin does not undergo significant metabolism | DrugBank |
| Excretion | ~89% of plazomicin is excreted via urine, ~0.2% is excreted via the feces | DrugBank |
| AMES Test (Carcinogenic Effect) | N/A | N/A |
| hERG Safety Test (Cardiac Effect) | N/A | N/A |
| Liver Toxicity | Aminoglycosides are not listed or mentioned in large case series of drug-induced liver disease and acute liver failure. Thus, hepatic injury due to plazomicin is rare if it occurs at all. | LiverTox |
Drug Interactions and Side Effects
Table 4. Drug interactions and side effects of plazomicin.
| Features | Comment(s) | Source |
|---|---|---|
| Total Number of Drug Interactions | 185 drugs | Drugs.com |
| Major Drug Interactions | 58 drugs (ex: bcg, diatrizoate, furosemide, mannitol) | Drugs.com |
| Alcohol/Food Interactions | No major alcohol or food interactions | Drugs.com |
| Disease Interactions | Neuromuscular disease (major) Hearing impairment (major) Renal dysfunction (major) Clostridioides difficile-associated diarrhea (CDAD) (major) Hepatic impairment (moderate) | Drugs.com |
| On-Target Side Effects | N/A | N/A |
| Off-Target Side Effects | Blood in the urine, change in the frequency of urination or amount of urine, difficulty in breathing, drowsiness, increased thirst, loss of appetite, nausea, swelling of the feet or lower legs, vomiting, ototoxicity | Drugs.com |
| CYP Interactions | N/A | N/A |
Links
Table 5. Links to learn more about plazomicin
| Comprehensive Antibiotic Resistance Database (CARD) | ARO:3003675 |
| DrugBank | DB12615 |
| Drugs.com | https://www.drugs.com/mtm/plazomicin.html |
| FDA - Zemdri | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210303Orig1s000lbl.pdf |
| LiverTox: National Institutes of Health (NIH) | https://www.ncbi.nlm.nih.gov/books/NBK548540/ |
| PubChem CID | 42613186 |
Learn about plazomicin resistance.
References
Golkar, T., Bassenden, A. V., Maiti, K., Arya, D. P., Schmeing, T. M., & Berghuis, A. M. (2021). Structural basis for plazomicin antibiotic action and resistance. Communications biology, 4(1), 1-8. https://doi.org/10.1038/s42003-021-02261-4
Jia, B., Raphenya, A. R., Alcock, B., Waglechner, N., Guo, P., Tsang, K. K., Lago, B. A., Dave, B. M., Pereira, S., Sharma, A. N., Doshi, S., Courtot, M., Lo, R., Williams, L. E., Frye, J. G., Elsayegh, T., Sardar, D. Westman, E. L., Pawlowski, A. C., Johnson, T. A., Brinkman, F. S., Wright, G. D., and McArthur, A. G. (2017) CARD 2017: expansion and model-centric curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Research 45, D566-573. https://doi.org/10.1093/nar/gkac920
Krause, K. M., Serio, A. W., Kane, T. R., & Connolly, L. E. (2016). Aminoglycosides: An Overview. Cold Spring Harbor perspectives in medicine, 6(6), a027029. https://doi.org/10.1101/cshperspect.a027029
LiverTox - Clinical and Research Information on Drug-Induced Liver Injury. National Institutes of Health. https://www.ncbi.nlm.nih.gov/books/NBK548540/
Plazomicin. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Plazomicin
Plazomicn - DrugBank. Drugbank.ca https://go.drugbank.com/drugs/DB12615
Plazomicin (injection). Drugs.com https://www.drugs.com/mtm/plazomicin.html
Zemdri (plazomicin for injection) (2018) Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210303Orig1s000lbl.pdf
March 2025, Helen Gao, Shuchismita Dutta; Reviewed by Drs. Albert Berghuis and Tolou Golkar
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/prot-syn/ribo/AMG/plazomicin