Plazomicin Resistance
Susceptibility Testing
Like all other antibiotics, the effectiveness of plazomicin against various infectious pathogens needs to be monitored. Since plazomicin was approved by the US FDA for clinical use in 2018, various studies to assess its minimum inhibitory concentrations (MICs) continue to be studied using various methods (Blanchard et al., 2022). Plazomicin provides a treatment option for complicated urinary tract infections with multidrug-resistant Enterobacterales, and resistance is rare. However, in metallo-β-lactamase-producing and carbapenem-resistant Enterobacterales, presence of a 16S rRNA methyltransferase can lead to plazomicin resistance. Hence the MICs should be regularly monitored.
Resistance Mechanisms
Like other aminoglycosides, plazomicin may display resistance due to the following mechanisms:
* Antibiotic target alteration
* Antibiotic Inactivation
Antibiotic target alteration
The ribosome (target) may be altered either by mutations or chemical modifications (e.g., methylation). Mutation or methylation of specific bases in the 16S rRNA which directly or indirectly interact with the antibiotic plazomicin, can have a big impact on antibiotic binding and lead to resistance.
Learn more about the methylation of ribosomal targets.
Antibiotic Inactivation
Plazomicin was designed to evade most clinically relevant aminoglycoside modifying enzymes, which contribute to the main resistance mechanism of aminoglycosides (Figure 4). It can avoid inactivation by AAC(3) and AAC(6′), the most common aminoglycoside acetyltransferases in P. aeruginosa. It is also resistant to ANT(2") and APH(2"), the most common adenyltransferase and phosphotransferase in the Enterobacteriaceae family (Golkar et al., 2021). Plazomicin also lacks hydroxyl groups at the 3′ and 4′ positions, protecting it against the activity of AMEs ANT(4′) and APH(3′). However, other studies have identified aminoglycoside modifying enzymes that can act on the 2' and 2" positions of plazomicin (Cox et al., 2018). Here we examine the structures of two AMEs in complex with plazomicin - APH(2")-IVa (Cox et al., 2018) and AAC(2')-Ia (Golkar et al., 2021).
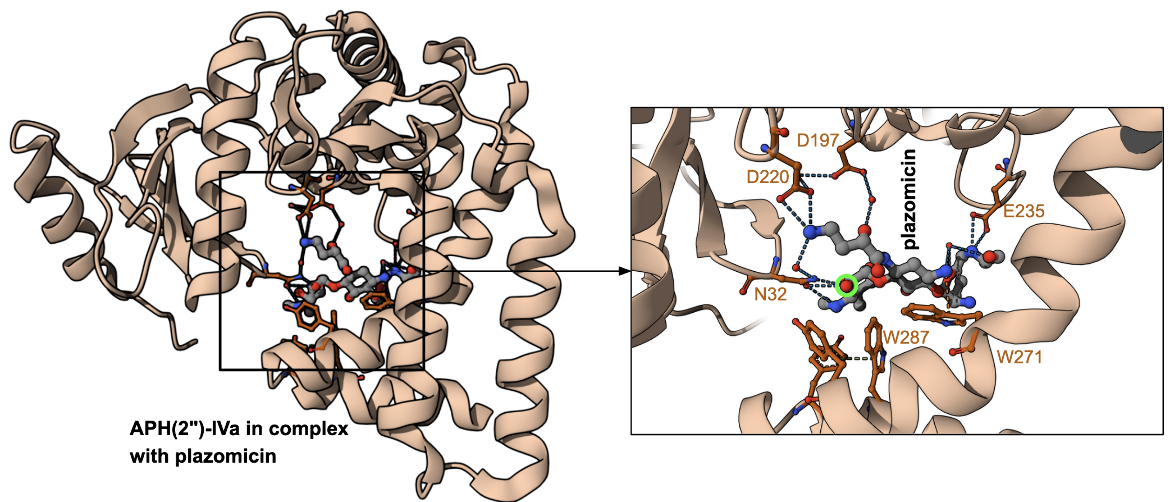
APH(2")-IVa resistance enzyme
The structure of aminoglycoside phosphotransferase (2")-IVa from Enterococcus casseliflavus in complex with plazomicin shows the antibiotic bound to the enzyme active site (Figure 4). Plazomicin’s core deoxystreptamine is packed against the sidechains of Trp271 and Trp287. The 2"-OH and 3″-N of plazomicin interact with Asn32 but these are far away from the catalytic Asp197. The site of modification is marked with a fluorescent green circle.
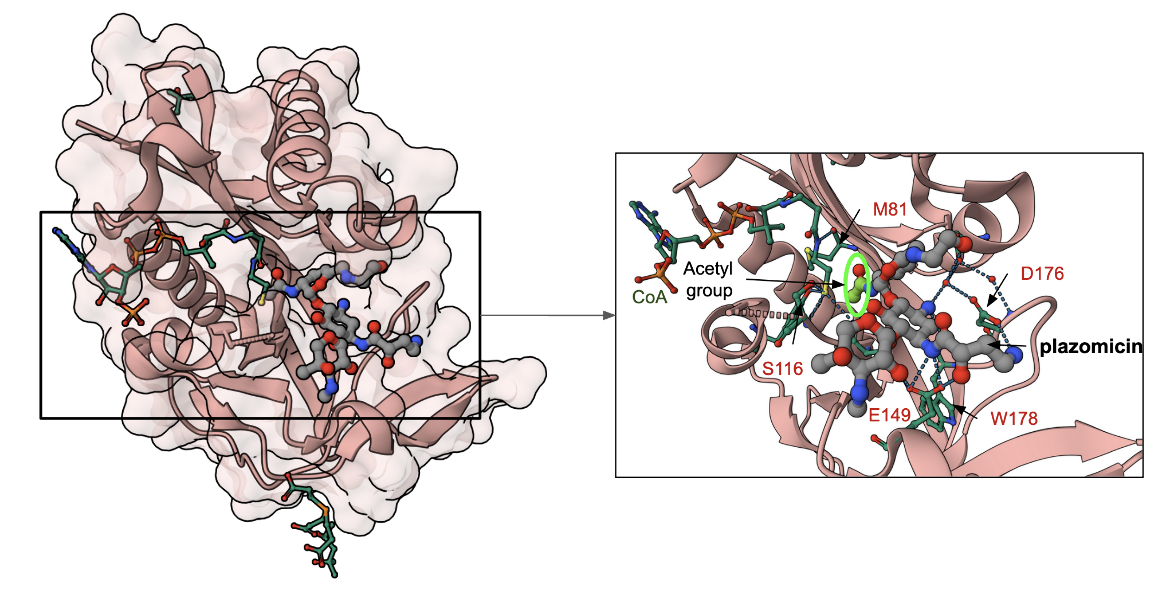
AAC(2′)-Ia resistance enzyme
The crystals of Aminoglycoside N-2'-Acetyltransferase-Ia from Providencia stuartii were grown in the presence of the substrates plazomicin and acetyl-CoA. The electron density maps following structure determination showed that the enzyme had covalently modified plazomicin by transferring an acetyl group from the cofactor leaving acetylated plazomicin in the enzyme active site and CoA (PDB ID 6vou, Golkar et al., 2021) is shown in Figure 5. The central ring of the antibiotic forms hydrogen bonds with the carboxy-terminal Trp178, while the N1 secondary amine moiety forms hydrogen bonds with Glu149 and Asp176. The N6′ extension forms a hydrogen bond with residues Asp32, while Ser114 interacts with the 2′-amine, while residues Ala80 (not shown in Figure 5) and Met81 interact with the oxygen of the 2′-acetyl modification.
Learn more about aminoglycoside modifying enzymes in general.
Back to the article on plazomicin.
References
Blanchard, L. S., Van Belkum, A., Dechaume, D., Armstrong, T. P., Emery, C. L., Ying, Y. X., Kresken, M., Pompilio, M., Halimi, D., Zambardi, G. (2022) Multicenter Clinical Evaluation of ETEST Plazomicin (PLZ) for Susceptibility Testing of Enterobacterales. J Clin Microbiol. 60(1):e0183121. https://doi.org/10.1128/jcm.01831-21
Cox, G., Ejim, L., Stogios, P. J., Koteva, K., Bordeleau, E., Evdokimova, E., Sieron, A. O., Savchenko, A., Serio, A. W., Krause, K. M., Wright, G. D. (2018) Plazomicin Retains Antibiotic Activity against Most Aminoglycoside Modifying Enzymes. ACS Infect Dis. 2018 Jun 8;4(6):980-987. https://doi.org/10.1021/acsinfecdis.8b00001
Golkar, T., Bassenden, A. V., Maiti, K., Arya, D. P., Schmeing, T. M., Berghuis, A. M. (2021) Structural basis for plazomicin antibiotic action and resistance. Commun Biol. 2021 Jun 11;4(1):729. https://doi.org/10.1038/s42003-021-02261-4