Clavulanic Acid
Drug Name
Clavulanic acid is a β-lactamase inhibitor derived from the organism Streptomyces clavuligerus and used to enhance the effectiveness of β-lactam antibiotics. It is frequently combined with amoxicillin or ticarcillin to fight antibiotic resistance by preventing their degradation by β-lactamase enzymes, broadening their spectrum of susceptible bacterial infections.
Table 1. Basic profile of clavulanic acid.
| Description | Orally or intravenously administered β-lactamase inhibitor |
| Target(s) | β-lactamases |
| Generic | Clavulanic acid |
| Commercial Name | Augmentin, Clavulin |
| Combination Drug(s) | Augmentin (Amoxicillin and Clavulanic Acid); Timentin® (ticarcillin/clavulanate). |
| Other Synonyms | Clavulanate |
| IUPAC Name | (2R,3Z,5R)-3-(2-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| Ligand Code in PDB | J01, L4A (Clavulanic acid, open form) |
| PDB Structure | 6NVU (Clavulanic Acid bound to TLA-1 extended spectrum β-lactamase) |
|
|
|
Inhibitor Chemistry
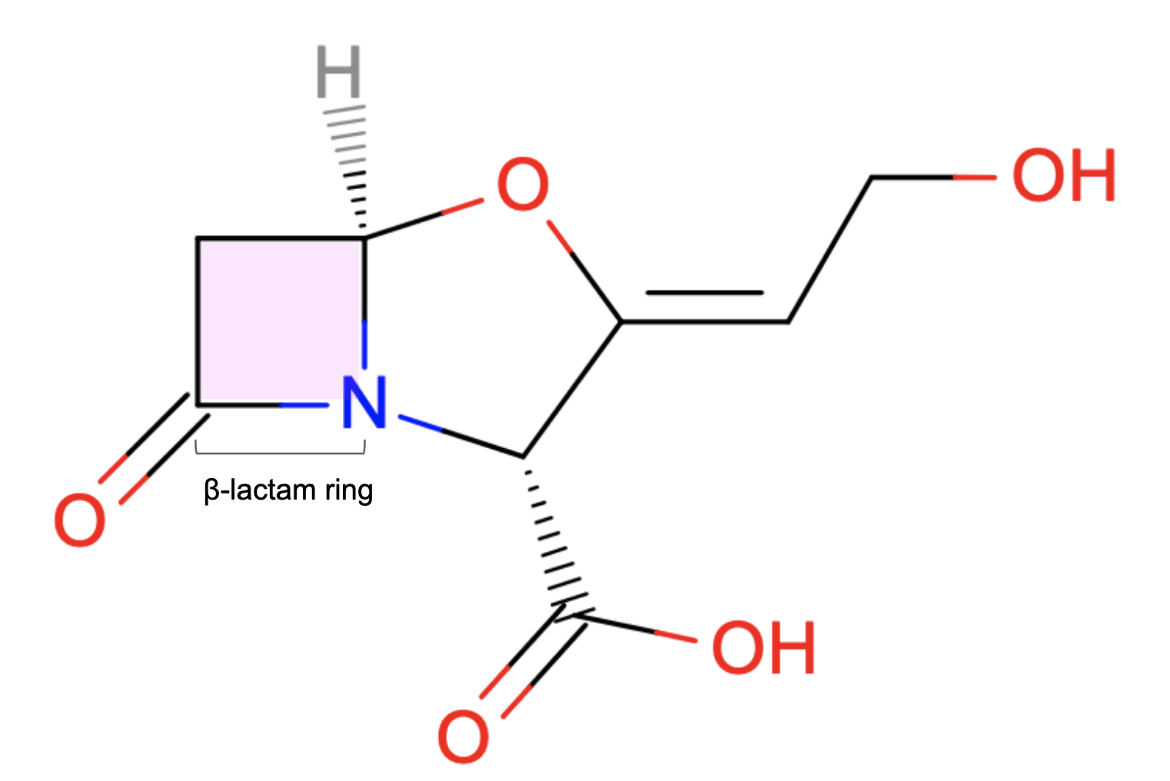
Clavulanic acid has an oxazolidine ring linked to the β-lactam ring (Figure 2). Although not an antibiotic, it can inhibit β-lactamases and is therefore used as an adjuvant or helper.
|
| Figure 2. 2D structure of Clavulanic acid showing the β-lactam ring responsible for its anti-β-lactamase activity. Structure created using ChemAxon. |
Drug Information
Table 2. Chemical and physical properties (DrugBank)
| Chemical Formula | C8H9NO5 |
| Molecular Weight | 199.16 g/mol |
| Calculated Predicted Partition Coefficient (cLogP) | -1.2 |
| Calculated Predicted Aqueous Solubility (cLogS) | 0.23 |
| Solubility (in water) | 337.0 mg/mL |
| Predicted Topological Polar Surface Area (TPSA) | 87.07 Å2 |
Drug Target
Clavulanic acid targets β-lactamase enzymes. These bacterial enzymes hydrolyze the amide bond of the β-lactam ring in β-lactam antibiotics, rendering them unable to inhibit their target enzyme. Learn more about β-lactamases.
Clavulanic acid mimics the interactions between β-lactam antibiotics and β-lactamases. When it is used in combination with β-lactam antibiotics, clavulanic acid inhibits β-lactamases from degrading the drug, acting as an effective mechanism against resistance to meropenems. The target enzyme TLA-1 β-lactamase is discussed here.
Drug-Target Complex
One target of clavulanic avid, TLA-1 β-lactamase, is an Ambler class A β-lactamase. TLA-1 β-lactamases are clinically important and are resistant to expanded-spectrum drugs including aztreonam, cephalosporins, and more.
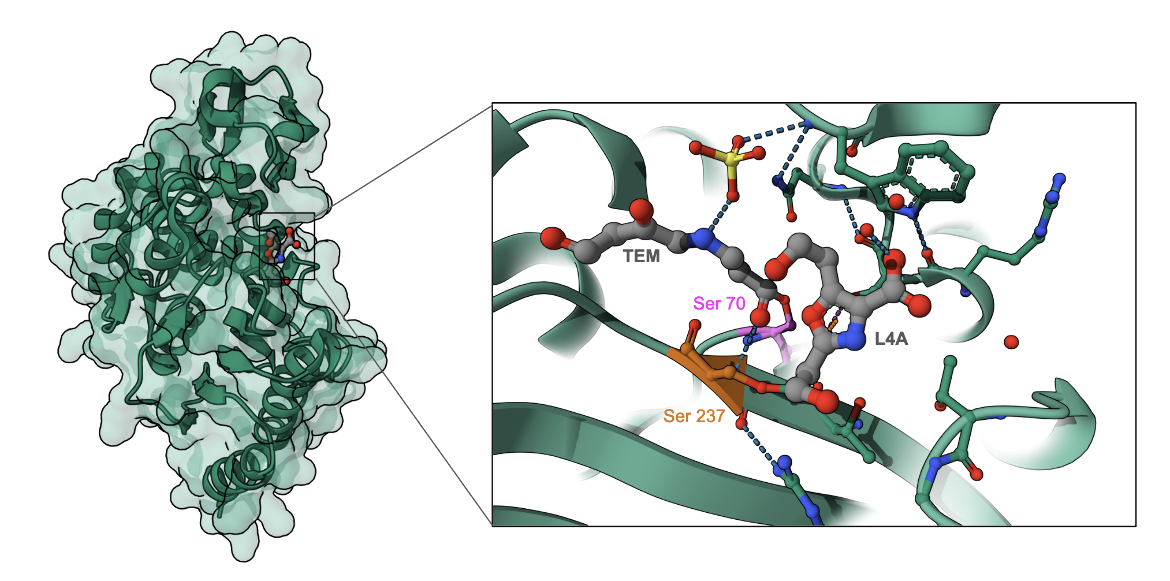
TLA-1 cleaves the amide bond of a β-lactam drug in 2 steps: acylation and deacylation. The oxygen of the Ser70 residue in TLA-1 attacks the carbonyl atom, and causes acylation of the β-lactam ring to form an acyl intermediate. This initiates a cascade of proton transfers, ultimately resulting in the cleavage of the amide bond. Deacylation regenerates the catalytic serine residue, releasing the hydrolyzed antibiotic.
Clavulinic acid (see Figure 3) forms a covalent adduct with the catalytic Ser70, shown as the trans-enamine intermediary form of the molecule (TEM), mimicking the transition state of the acylation and deacylation pathway. This blocks the active site of TLA-1 from cleaving any β-lactam antibiotic. This trans-enamine intermediate has both rings of the clavulanate opened. In addition, a single-ring form of the molecule (L4A) covalently links to another serine (Ser237) in the binding pocket (PDB ID 6nvu, Cifuentes-Castro et al., 2020).
Pharmacologic Properties and Safety
Table 3. Pharmacokinetics: ADMET of Augmentin (clavulanic acid and amoxicillin).
| Features | Comment(s) | Source |
|---|---|---|
| Oral Bioavailability (%) | 64% | DrugBank |
| IC50 | N/A | N/A |
| Ki (µM) | N/A | N/A |
| Half-life (hrs) | 0.75 - 1.5 hours | DrugBank |
| Duration of Action | 8-12 hours | LiverTox |
| Absorption Site | Gastrointestinal tract | DrugBank |
| Transporter(s) | N/A | N/A |
| Metabolism | Clavulanic acid is heavily metabolized to form the metabolites 2,5-dihydro-4-(2- hydroxyethyl)-5-oxo-1H-pyrrole-3-carboxylic acid and 1-amino-4-hydroxy-butan-2-one. The first metabolite was found to account for 15.6% of the dose while the second metabolite was reported to account for 8.8% of the dose in one pharmacokinetic study. | DrugBank |
| Excretion | The average clearance of clavulanic acid is 12.20 liters/h/70 kg. Dose adjustments may be required in patients with renal failure. | DrugBank |
| AMES Test (Carcinogenic Effect) | Non AMES toxic | DrugBank |
| hERG Safety Test (Cardiac Effect) | Weak inhibitor | DrugBank |
| Liver Toxicity | Amoxicillin-clavulanate has been implicated in hundreds of cases of clinically apparent acute liver injury and this combination is currently the most common cause of drug-induced liver disease in the United States and Europe. The onset of injury is typically a few days to as long as 8 weeks (average ~3 weeks) after initiation of therapy and often occurs after the course of the antibiotic is completed, the delay being a few days to as long as six weeks. | LiverTox |
Drug Interactions and Side Effects
Table 4. Drug interactions and side effects of clavulanic acid.
| Features | Comment(s) | Source |
|---|---|---|
| Total Number of Drug Interactions | 62 drugs | Drugs.com |
| Major Drug Interaction(s) | bcg (Tice BCG, Tice BCG Vaccine); cholera vaccine, live; leflunomide; lomitapide; methotrexate; mipomersen; pexidartinib; teriflunomide; typhoid vaccine, live | Drugs.com |
| Alcohol/Food Interaction(s) | N/A | N/A |
| Disease Interaction(s) | Liver disease (major) Colitis (major) Mononucleosis (moderate) Diabetes (moderate) Phenylketonuria (moderate) Renal dysfunction (moderate) Hemodialysis (moderate) | Drugs.com |
| On-target Side Effects | N/A | N/A |
| Off-target Side Effects | Nausea, vomiting, diarrhea, rash, itching, vaginal itching or discharge, diaper rash | Drugs.com |
| CYP Interactions | N/A | N/A |
Regulatory Approvals/Commercial
Clavulanic acid is frequently combined with Amoxicillin, Ticarcillin, or ceftazidime to prevent the drug from being degraded by β-lactamases. The combination of clavulanic acid with amoxicillin is commonly known as Augmentin. Learn more about Amoxicillin.
Links
Table 5. Links to learn more about clavulanic acid
| Comprehensive Antibiotic Resistance Database (CARD) | ARO: 0000079 |
| DrugBank | DB00766 |
| Drugs.com | https://www.drugs.com/international/clavulanic-acid.html |
| FDA | https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050564s049,050720s022lbl.pdf |
| LiverTox: National Institutes of Health (NIH) | LiverTox |
| PubChem CID | 5280980 |
References
Cifuentes-Castro, V., Rodríguez-Almazán, C., Silva-Sánchez, J., Rudiño-Piñera, E. (2020). The crystal structure of ESBL TLA-1 in complex with clavulanic acid reveals a second acylation site. Biochemical and biophysical research communications, 522(2), 545-551. https://doi.org/10.1016/j.bbrc.2019.11.138
Clavulanic acid. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050564s049,050720s022lbl.pdf
Clavulanic acid. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Clavulanic-acid
Clavulanic acid - DrugBank. https://go.drugbank.com/drugs/DB00766
Clavulanic acid. Drugs.com https://www.drugs.com/international/clavulanic-acid.html
Jia, B., Raphenya, A. R., Alcock, B., Waglechner, N., Guo, P., Tsang, K. K., Lago, B. A., Dave, B. M., Pereira, S., Sharma, A. N., Doshi, S., Courtot, M., Lo, R., Williams, L. E., Frye, J. G., Elsayegh, T., Sardar, D. Westman, E. L., Pawlowski, A. C., Johnson, T. A., Brinkman, F. S., Wright, G. D., McArthur, A. G. (2017) CARD 2017: expansion and model-centric curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Research 45, D566-573. https://doi.org/10.1093/nar/gkw1004
April 2025, Helen Gao and Shuchismita Dutta; Reviewed by Dr. Gregg Crichlow
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/OR/inh-blmase/clavulanicA