Vaborbactam
Drug Name
Vaborbactam is a β-lactamase inhibitor used to treat bacterial infections in patients with renal disease. It is used in combination with meropenem, a semi-synthetic broad-spectrum antibacterial drug, under the name Vabomere. Although vaborbactam has no antibacterial activity alone, it inhibits β-lactamases from degrading meropenem, acting as an effective mechanism against resistance to meropenem by bacteria.
Table 1. Basic profile of vaborbactam.
| Description | Intravenously (IV) administered drug that restores the antibacterial activity of other antibiotic drugs |
| Target(s) | β-lactamases |
| Generic | Vabobactam |
| Commercial Name | Vabomere, Vaborem |
| Combination Drug(s) | Vabomere® (meropenem & vabobactam; United States) |
| Other Synonyms | N/A |
| IUPAC Name | 2-[(3R,6S)-2-hydroxy-3-[2-(thiophen-2-yl)acetamido]-1,2-oxaborinan-6-yl]acetic acid |
| Ligand Code in PDB | 4D6 |
| PDB Structures | 4xux (Vaborbactam bound to ampC), 4xuz (Vaborbactam bound to CTX-M-15) |
|
|
|
Inhibitor Chemistry
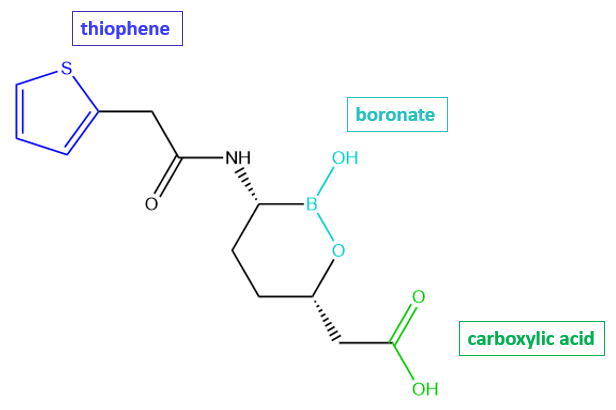
Although vaborbactam inhibits β-lactamases, it does not have a β-lactam ring. Instead, it has a cyclic boronate that forms a reversible covalent bond with the active site Ser in Ambler Class A β-lactamases.
|
| Figure 2. 2D structure of vaborbactam showing the functional moieties responsible for its anti-β-lactamase activity. Structure created using ChemDraw. |
Drug Information
Table 2. Chemical and physical properties (DrugBank).
| Chemical Formula | C12H16BNO5S |
| Molecular Weight | 297.13 g/mol |
| Calculated Predicted Partition Coefficient (cLogP) | 1.02 |
| Calculated Predicted Aqueous Solubility (cLogS) | -3.3 |
| Solubility (in water) | 0.155 mg/mL |
| Predicted Topological Polar Surface Area (TPSA) | 95.86 Å2 |
Drug Target
Vaborbactam targets β-lactamase enzymes. These bacterial enzymes hydrolyze the amide bond of the β-lactam ring in β-lactam antibiotics, rendering them unable to inhibit their target enzyme. Learn more about β-lactamases.
The inhibitor vaborbactam is used in combination with meropenem to inhibit β-lactamases from degrading the antibiotic, as an effective mechanism against resistance to meropenem by bacteria. Vaborbactam mimics the interactions between meropenem and β-lactamases, thereby preventing the β-lactamases from degrading meropenem.
Vaborbactam interacts with Ambler classes A and C β-lactamases via covalent binding. It has no inhibitory actions on class D or class B carbapenemases. Thus, the targets of vaborbactam CTX-M-15 (class A β-lactamase) and AmpC (class C β-lactamase), will be discussed here.
Drug-Target Complex
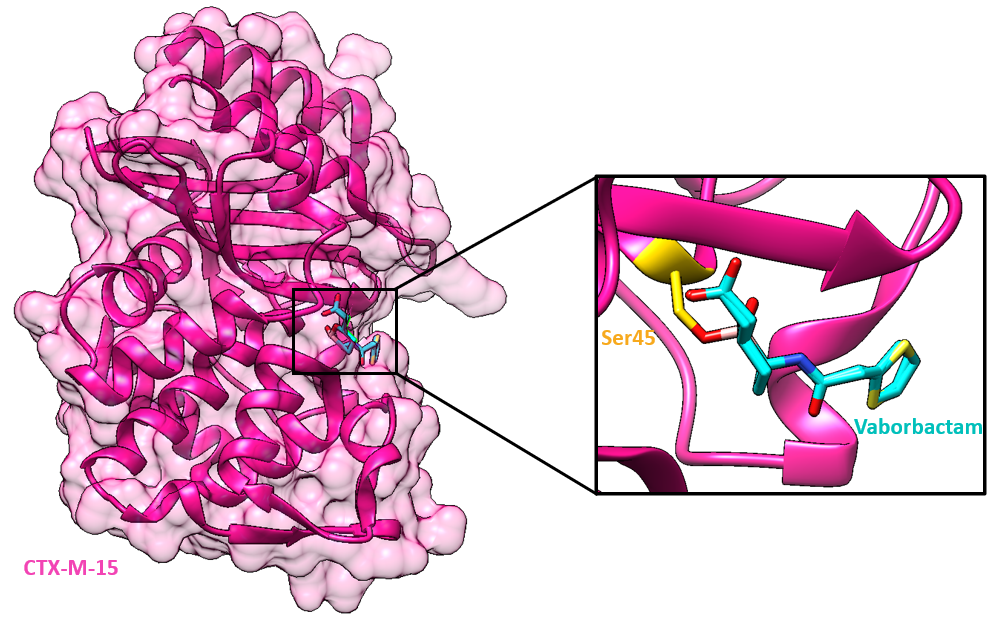
One target of vaborbactam, CTX-M-15, is an Ambler class A β-lactamase. CTX-M β-lactamases are clinically important and were named for their enhanced activity against the third-generation of cephalosporin cefotaxime (Jia et al., 2017). CTX-M genes have been found on many kinds of mobile genetic elements which can be transmitted to other bacteria. Consequently, CTX-M genes have become the most prevalent extended-spectrum β-lactamase and have greater potential to spread beyond hospital environments compared to other β-lactamases.
CTX-M-15 cleaves the amide bond of a β-lactam drug in 2 steps: acylation and deacylation. The oxygen of the Ser45 residue in CTX-M-15 attacks the carbonyl atom, and causes acylation of the β-lactam ring to form an acyl intermediate. This initiates a cascade of proton transfers, ultimately resulting in the cleavage of the amide bond. Deacylation regenerates the catalytic serine residue, releasing the hydrolyzed antibiotic.
Vaborbactam's boronate atom (see Figure 3) forms a covalent adduct with the catalytic Ser45, mimicking the transition state of the acylation and deacylation pathway. The Ser45 corresponds to Ser70 in canonical class A beta-lactamase residue numbering. The formation of this covalent adduct blocks the active site of CTX-M-15 from cleaving the antibiotic meropenem.
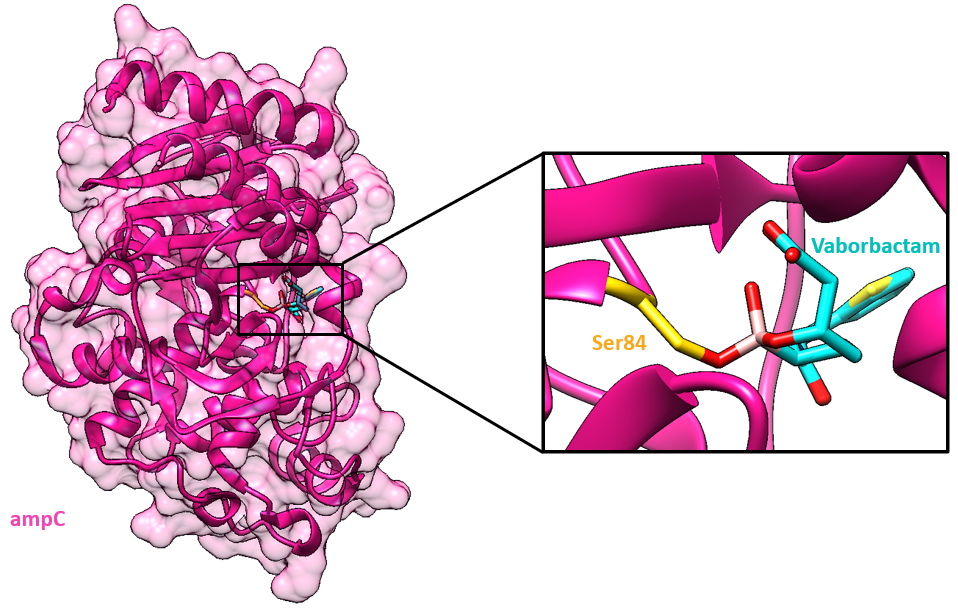
Another target of vaborbactam, ampC, is an Ambler class C β-lactamase. Similar to CTX-M β-lactamases, ampC uses a cataytic serine in the acylation step to break the amide bond in the β-lactam drug. This Serine is typically numbered Ser64 in canonical class C residue numbering, but in PDB entry 4xux, it is called Ser84. The mechanism of deacylation is still debated in the scientific community, though the result still regenerates the enzyme and releases the degraded drug.
Learn more about the function of ampC.
Vaborbactam has a boronate moiety (shown in aqua in Figure 4) that contributes to its ability to inhibit the catalytic serine residue in β-lactamases.
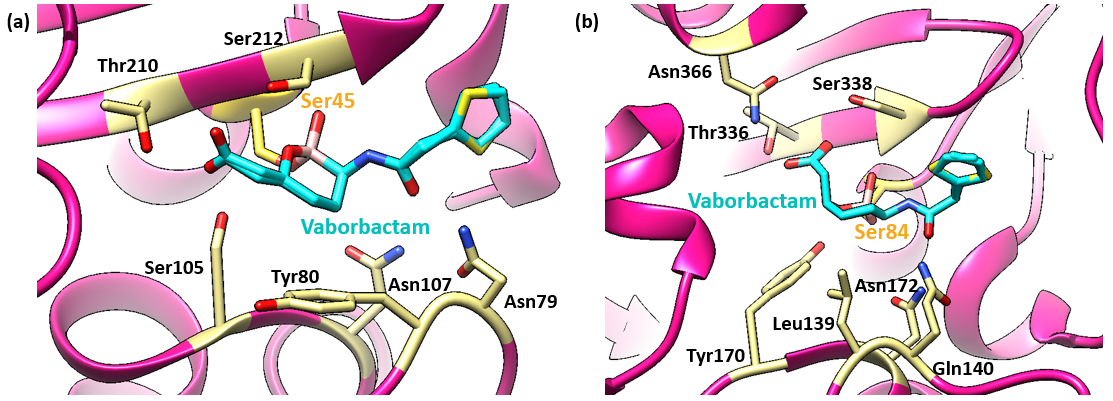
The structures of CTX-M-15 (Figure 5a) and ampC (Figure 5b) complexes clearly show that the catalytic serine residue of each enzyme is covalently bound to the boron atom of the inhibitor. Other common features exist. In each, the amide moiety of the inhibitor is extensively coordinated, donating a hydrogen bond to the Ser212/ Ser338 backbone carbonyl and accepting hydrogen bonds from the side chains of Asn132/ Asn152 and Asn79/ Gln140 (CTX-M-15/AmpC residue numbers, in 4xuz and 4xux, respectively). The carboxylate of the ligand is coordinated by Thr210/ Thr336, Ser212/ Ser338, and Ser 105 (CTX-M-15 only) side chain hydroxyls.
The structures of CTX-M-15 and ampC also differ remarkably in the active site. The vaborbactam molecules in both structures are in the "chair" conformation. However, in the CTX-M-15 complex the amide substituent is in the axial orientation and carboxymethyl is equatorial, while in the AmpC complex the amide is equatorial and carboxymethyl is axial.
The conformation of vaborbactam in ampC allows for a more substrate-like interaction with the enzyme, with the hydroxyl oxygen of the boronate interacting in the oxyanion hole formed by the backbone NH groups of Ser84 and Ser338, while the oxygen in the boronate ring forms a hydrogen bond to the hydroxyl of Tyr170. These two oxygen atoms likely correspond to the carbonyl oxygen and the nitrogen of the β-lactam substrate, respectively. In CTX-M-15, both oxygen atoms of vaborbactam interact with the oxyanion hole, which is significantly expanded. This makes the axial configuration of the amide sterically preferable in CTX-M-15, compared to the axial positioning of the carboxymethyl group in ampC.
The ability of vaborbactam to switch between two conformations allows it to retain activity against two different β-lactamase classes.
Pharmacologic Properties and Safety
Table 3. Pharmacokinetics: ADMET of vaborbactam.
| Features | Comments | Source |
|---|---|---|
| Oral Bioavailability (%) | <1% | DrugBank |
| IC50 | 5 µM (for binding to AmpC) | (Langley et al., 2019) |
| Ki (µM) | 0.030 µM (Ki of vaborbactam inhibiting CTX-M-15); 0.035 µM (Ki of vaborbactam inhibiting AmpC) | (Tsivkovski & Lomovskaya, 2020) |
| Half-life (hrs) | 1.68 - 2.25 hours | DrugBank |
| Duration of Action | N/A | N/A |
| Absorption Site | N/A | N/A |
| Transporter(s) | N/A | N/A |
| Metabolism | Vaborbactam does not undergo metabolism. | DrugBank |
| Excretion | ~ 75%-95% of vaborbactam is excreted unchanged in the urine over a 24 to 48 hour period. | FDA |
| AMES Test (Carcinogenic Effect) | N/A | N/A |
| hERG Safety Test (Cardiac Effect) | N/A | N/A |
| Liver Toxicity | Vaborbactam does not undergo hepatic metabolism. Therefore, the systemic clearance of meropenem and vaborbactam is not expected to be affected by or affect the liver | FDA |
Drug Interactions and Side Effects
Table 4. Drug interactions and side effects of vaborbactam.
| Features | Comment(s) | Source |
|---|---|---|
| Total Number of Drug Interactions | 29 drugs | Drugs.com |
| Major Drug Interaction(s) | bcg (Tice BCG, Tice BCG Vaccine); bupropion; cholera vaccine, live; divalproex sodium; iohexol; iopamidol; metrizamide; tramadol; typhoid vaccine, live; valproic acid | Drugs.com |
| Alcohol/Food Interaction(s) | N/A | N/A |
| Disease Interaction(s) | Hypertension (moderate) | Drugs.com |
| On-target Side Effects | Phlebitis/thrombophlebitis at the injection site, infusion site thrombosis or erythema | Drugs.com |
| Off-target Side Effects | Diarrhea, nausea, headache, vomiting, hypersensitivity to drug, hypoglycemia, hypokalemia, hypotension | Drugs.com |
| CYP Interactions | N/A | N/A |
Regulatory Approvals/Commercial
Vaborbactam is used with meropenem to prevent the drug from being degraded by β-lactamases and increase its antibacterial properties. Learn more about meropenem.
Links
Table 5. Links to learn more about vaborbactam
| Comprehensive Antibiotic Resistance Database (CARD) | 3004380 |
| DrugBank | DB12107 |
| Drugs.com | Vabomere: https://www.drugs.com/mtm/vabomere.html |
| FDA | FDA" target="_blank">https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209776lbl.pdf |
| LiverTox: National Institutes of Health (NIH) | N/A |
| PubChem CID | 56649692 |
References
Hecker, S. J., Reddy, K. R., Totrov, M., Hirst, G. C., Lomovskaya, O., Griffith, D. C., King, P., Tsivkovski, R., Sun, D., Sabet, M., Tarazi, Z., Clifton, M. C., Atkins, K., Raymond, A., Potts, K. T., Abendroth, J., Boyer, S. H., Loutit, J. S., Morgan, E. E., Durso, S., Dudley, M. N. (2015). Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class A serine carbapenemases. https://doi.org/10.1021/acs.jmedchem.5b00127
Jia, B., Raphenya, A. R., Alcock, B., Waglechner, N., Guo, P., Tsang, K. K., Lago, B. A., Dave, B. M., Pereira, S., Sharma, A. N., Doshi, S., Courtot, M., Lo, R., Williams, L. E., Frye, J. G., Elsayegh, T., Sardar, D. Westman, E. L., Pawlowski, A. C., Johnson, T. A., Brinkman, F. S., Wright, G. D., and McArthur, A. G. (2017) CARD 2017: expansion and model-centric curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Research 45, D566-573. https://doi.org/10.1093/nar/gkw1004
Langley, G. W., Cain, R., Tyrrell, J. M., Hinchliffe, P., Calvopiña, K., Tooke, C. L., Widlake, E., Dowson, C. G., Spencer, J., Walsh, T. R., Schofield, C. J., Brem, J. (2019) Profiling interactions of vaborbactam with metallo-β-lactamases. Bioorg Med Chem Lett. 29, 1981-1984. https://doi.org/10.1016/j.bmcl.2019.05.031
Tsivkovski, R., Lomovskaya, O. (2020). Biochemical activity of vaborbactam. Antimicrobial agents and chemotherapy, 64(2), e01935-19. https://doi.org/10.1128/aac.01935-19
Vaborbactam. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209776lbl.pdf
Vaborbactam. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/56649692
Vaborbactam - DrugBank. Drugbank.ca http://www.drugbank.ca/drugs/DB12107
Vabomere. Drugs.com https://www.drugs.com/mtm/vabomere.html
April 2025, Helen Gao; Reviewed by Dr. Gregg Crichlow
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/OR/inh-blmase/vaborbactam