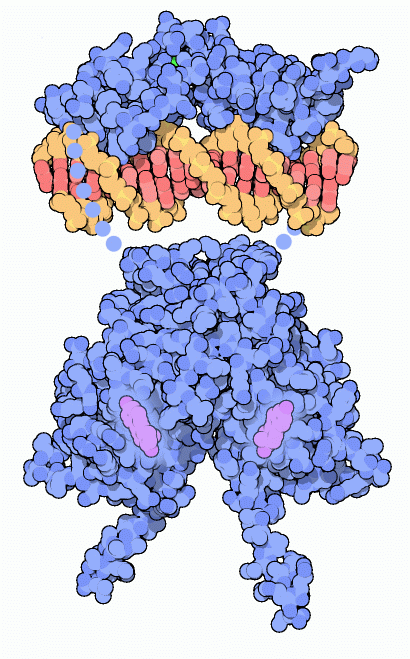
Estrogen receptor bound to a short piece of DNA. Flexible portions of the protein that are not included in the structures are shown schematically with dots.
Download high quality TIFF image
Our bodies grow in two major spurts during our lives. You probably don't remember the first, which occurred during the first few months of your life as you grew from a single cell into a fully formed baby. On the other hand, you probably have many memories (or you may be making those memories now) of your second frenzy of growth. During puberty, sex hormones direct selected tissues to undergo a second wave of growth and development. In women, estrogen hormones are largely responsible for coordinating these changes. Estrogens are made in the ovaries and then delivered within seconds throughout the body in the blood, directing the growth from child to adult.
Straight to the Source
Estrogens are small, carbon-rich molecules built from cholesterol. This is quite different than larger hormones, such as insulin and growth hormone, which are sensed by receptors on the cell surface. Estrogens pass directly into cells throughout the body, so the cell can use receptors that are in the nucleus, right at the site of action on the DNA. When estrogen enters the nucleus, it binds to the estrogen receptor, causing it to pair up and form a dimer. This dimer then binds to several dozen specific sites in the DNA, strategically placed next to the genes that need to be activated. Then, the DNA-bound receptor activates the DNA-reading machinery and starts the production of messenger RNA.
A Large Family
When researchers looked into the human genome, they found over 150 proteins that are similar to the estrogen receptor. This is a large family of nuclear receptors that sense the levels of small hormones and other signaling molecules, such as steroid and thyroid hormones, vitamin D, and retinoic acid. Like estrogen, these are all small molecules that pass directly into cells and find their way to the nucleus. These receptors each bind to a specific signaling molecule and then activate or repress their own set of 50-100 genes.
Recognizing Estrogens
The estrogen receptor, and other nuclear receptors, are composed of several parts connected into one long chain. The structures of two of these parts are available in the PDB. At one end is the part that binds to the hormone, shown at the bottom of the illustration from PDB entry
1a52 . This structure has estradiol bound, shown here in purple. This is connected to a DNA-binding domain that recognizes specific sequences of DNA, shown here at the top using coordinates in PDB entry
1hcq . Finally, there is a large transactivation domain attached at the end of the DNA-binding domain, which is not shown here. It activates RNA polymerase when the receptor binds to DNA.
Estrogen and Cancer
Estrogen gives cells permission to grow when appropriate. This is essential during puberty, but also necessary in adult life. For instance, estrogen is important in bone growth, and low levels of estrogens can lead to osteoporosis. But in the case of cancer, estrogen can enhance unnatural growth and make the disease worse. The drug tamoxifen is used to treat cancer by blocking the action of estrogen. Tamoxifen is a small drug that mimics the shape of estrogen and binds tightly to the estrogen receptor. When it binds, it changes the shape of a signaling loop on the surface the receptor, colored green here. The upper structure shown here, from PDB entry
1qku , has estradiol bound (estradiol is not seen in the picture, because it is bound under the loop). The compact conformation of this loop forms part of the activation signal that will stimulate normal growth. The lower structure, from PDB entry
3ert , has the drug bound. Since the drug is larger than the hormone, it forces the activation loop out into an inactive conformation, blocking the signal to grow.
You can look at the DNA-binding part of the estrogen receptor in PDB entry
1hcq . The receptor binds to DNA using two "zinc fingers." These are small domains built around zinc ions. Four cysteine amino acids (shown in yellow here) surround each zinc ion (shown in green), forming a sturdy core that gives the domain a rigid structure. The receptor places an alpha helix in the major groove of DNA--in this picture, we are looking straight down the helix. Several amino acids on one side of this helix reach out and grip the edges of the base pairs, making sure that the DNA is of the appropriate sequence.
This illustration was created with RasMol. You can create similar illustrations by clicking on the accession codes in this article and then picking one of the options for 3D viewing.






