Diaminopyrimidine
Discovery
Folate synthesis is critical in organisms ranging from bacteria to humans. However, the specificity of these biosynthetic enzymes in different organisms is slightly different. In the 1940s, George H. Hitchings and his associates synthesized many different analogues of 2,4-diaminopyrimidine (including Trimethoprim) as competitive inhibitors of the enzyme dihydrofolate reductase, catalyzing the last step in folate biosynthesis (Hitchings and Burchall, 1965; Then, 1993).
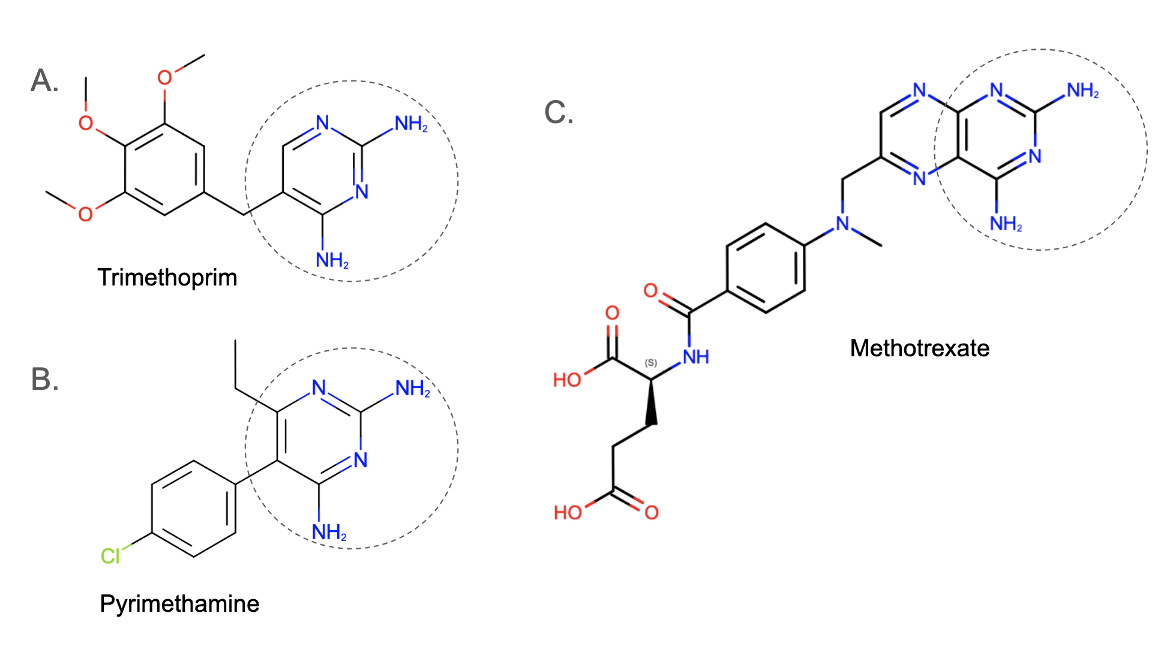
Using the various diaminopyrimidine analogues to test for specificity in different cases, trimethoprim (Figure 1A) was effective against bacteria (as an antibiotic); pyrimethamine (Figure 1B) was effective against protozoa (as an antimalarial drug); whereas methotrexate (Figure 1C) was found to have good activity against mammalian cell (as a chemotherapeutic agent to treat cancer) (van Miert, 1994).
George Hitchings shared the 1988 Nobel Prize in Physiology or Medicine with James Black and Gertrude Elion for their discoveries of important principles for drug treatment.
Overview of Chemistry
Diaminopyrimidines are a class of organic compounds containing a pyrimidine ring substituted by two amine groups (see portions marked by circles in dotted lines in Figure 1). They are inhibitors of dihydrofolate reductase, an enzyme critical for DNA synthesis and amino acid metabolism.
Learn more about folate synthesis.
|
| Figure 1. 2D chemical drawings of a diaminopyrimidines. A. Trimethoprim (CC ID TOP); B. Pyrimethamine (CC ID CP6); and C. Methotrexate (CC ID MTX). Drawing made using ChemAxon software. |
Types
The DHFR inhibitors are sometimes classified into two groups (Wróbel et al., 2020) - (a) classical inhibitors - which are folate analogues that have a heterocyclic ring bound to the aryl group and glutamate tail, and (b) nonclassical inhibitors - these have a lipophilic side chain. The lipophilic antifolates can passively diffuse into the cytosolic space, while classical antifolates like Methotrexate possess a glutamate moiety, which is negatively charged and must be actively transported into the cell via specific carriers. Bacteria do not have such transporters so they do not respond to these molecules (Reeve et al., 2019).
Methotrexate is an example of a classical inhibitor, while examples of non-classical inhibitors include trimethoprim and pyrimethamine (see Figure 1).
Resistance
Like most other antibiotics, resistance to diaminopyrimidines is also common. After its introduction in 1968, trimethoprim resistance was first seen in S. aureus in the 1980s (Reeve et al., 2019). The main mechanisms of resistance to trimethoprim include the production of additional plasmids-, transposons-, cassettes-mediated drug-resistant DHFR. Learn more about trimethoprim resistance.
Although several diaminopyrimidines were initially tested as monotherapies in clinical trials, pronounced synergism between some of these compounds and sulfonamides were found to be more effective as antimicrobials and antiprotozoals. Careful combinations of diaminopyrimidines and sulfonamides also reduced resistance development (van Miert, 1994). More recently, some clinicians have moved back to monotherapies to avoid the allergic reactions seen in sulfonamides. Learn more about sulfonamides.
References
Hitchings, G. H., Burchall, J. J. (1965) Inhibition of folate biosynthesis and function as a basis for chemotherapy. Adv Enzymol Relat Areas Mol Biol. 27, 417-68. https://doi.org/10.1002/9780470122723.ch9
Reeve, S. M., Si, D., Krucinska, J., Yan, Y., Viswanathan, K., Wang, S., Holt, G. T., Frenkel, M. S., Ojewole, A. A., Estrada, A., Agabiti, S. S., Alverson, J. B., Gibson, N. D., Priestley, N. D., Wiemer, A. J., Donald, B. R., Wright, D. L. (2019) Toward Broad Spectrum Dihydrofolate Reductase Inhibitors Targeting Trimethoprim Resistant Enzymes Identified in Clinical Isolates of Methicillin Resistant Staphylococcus aureus. ACS Infect Dis. 5(11):1896-1906. https://doi.org/10.1021/acsinfecdis.9b00222
Then R. L. (1993) History and future of antimicrobial diaminopyrimidines. J Chemother. 5(6):361-8. PMID: 8195827.
van Miert AS. (1994) The sulfonamide-diaminopyrimidine story. J Vet Pharmacol Ther. 17(4):309-16. https://doi.org/10.1111/j.1365-2885.1994.tb00251.x
Wróbel, A., Arciszewska, K., Maliszewski, D., Drozdowska, D. (2020) Trimethoprim and other nonclassical antifolates an excellent template for searching modifications of dihydrofolate reductase enzyme inhibitors. J Antibiot (Tokyo). 73(1):5-27. https://doi.org/10.1038/s41429-019-0240-6
March 2025, Shuchismita Dutta; Reviewed by Dr. Christina Bourne
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/folate-synth/DHR/diaminopyrimidine



