Incretins
Introduction
Type 2 diabetes may result from a variety of conditions including β-cell dysfunction, where insulin is not produced, and/or due to insulin resistance, in both cases leading to hyperglycemia, and complications in both small and large blood vessels (Silberstein, 2013). Over time, as liver, adipose and skeletal muscle tissues develop a reduced insulin sensitivity, insulin secretion from pancreatic β-cells is no longer suffice in maintaining blood glucose levels within the normal homeostatic range, resulting in hyperglycemia (Yamada et al., 2006). Many of the traditional oral antihyperglycemic agents (e.g. metformin, sulfonylureas, TZDs, etc.) address only one of the underlying pathophysiology - lowering postprandial glucose levels, but their safety profiles and side effects, such as weight gain and hypoglycemia, may present challenges and limitations in their clinical use. Therefore, a combination treatment regimen is often prescribed to target the multifaceted pathophysiology of type 2 diabetes. Recently, incretin-based therapies have emerged as a novel and promising treatment approach for patients as they enhance glycemic control through various mechanisms including promoting glucose-dependent pancreatic secretion of insulin in response to postprandial glucose levels (Joy, Rodgers and Scates, 2005). Unlike the other antidiabetic treatment options, incretin mimetics do not induce hypoglycemia and either cause weight loss or or no change in weight.
The Incretin Effect
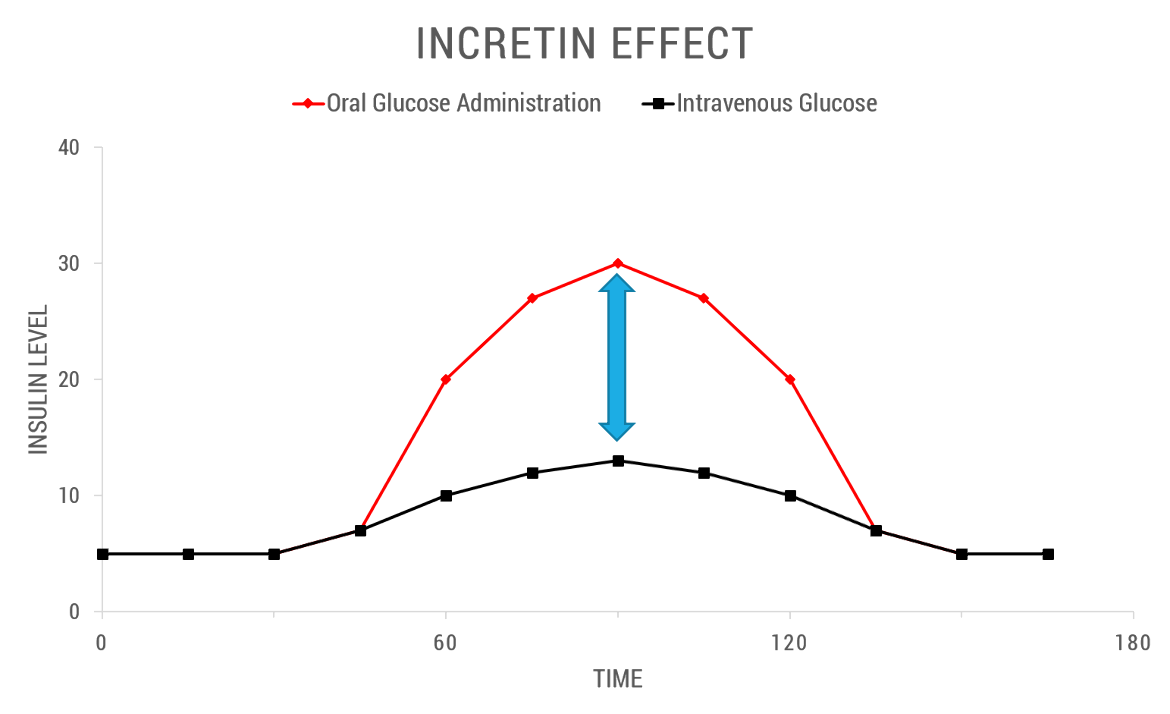
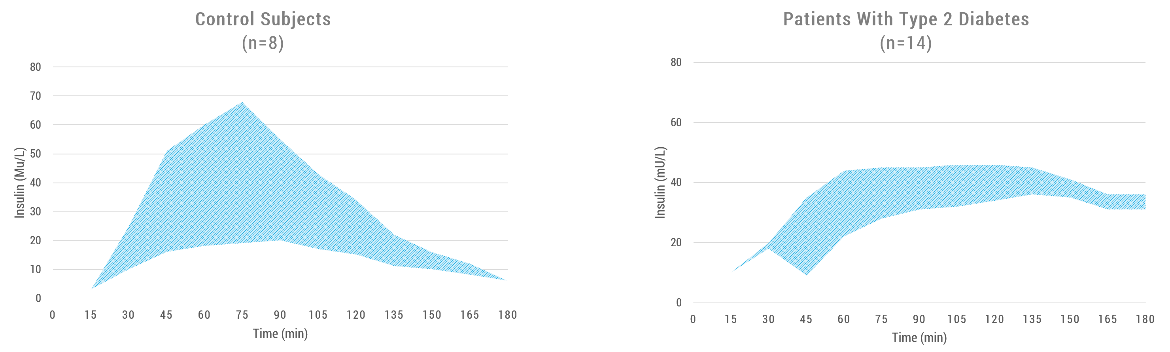
The incretin effect is a phenomenon whereby an oral glucose load provokes a greater insulin secretory response than the same amount of insulin released when glucose was injected intravenously. Figure 1 below depicts the incretin effect in a normal patient versus a diabetic patient.
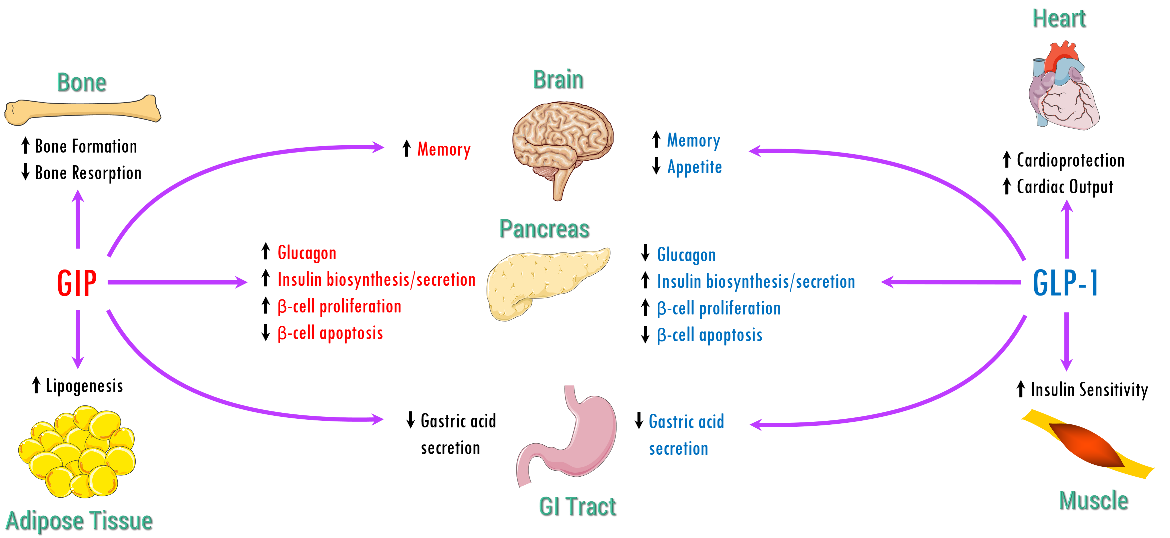
Two main incretin hormones have been identified: glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Within the pancreas, GIP and GLP act collectively to promote β-cell proliferation and inhibit apoptosis and also play critical roles in various biological processes in tissues and organs that express GIP receptor and GLP-1 receptor. Despite the similarities, these two hormones differ in secretion and metabolism, their insulinotropic action on pancreatic β-cells, and their non-insulinotropic effects (Seino, Fukushima and Yabe, 2010) as shown in Table 1.
Table 1. Overlapping and contrasting characteristics and actions of GLP-1 and GIP (Seino, Fukushima and Yabe, 2010).
| GLP-1 | GIP |
|---|---|
| 30 or 31 amino acid peptide | 42 amino acid peptide |
| Secreted from L-cells in ileum and colon | Secreted from K-cells in duodenum |
| Enhances glucose-stimulated insulin secretion in β-cells | Enhances glucose-stimulated insulin secretion in β-cells and glucagon in ɑ-cells during hyperglycemic and hypoglycemic conditions respectively |
| Inhibits hepatic glucagon secretion in a glucose-dependent manner | No significant inhibition of hepatic glucagon secretion |
| Potent inhibition of gastric emptying | Moderate effects on gastric emptying |
| Reduction of food intake and body weight | Insignificant effects on satiety or body weight; increases fat deposition |
| Significant effects on β-cell growth and survival | Potential effects on β-cell growth and survival |
| Insulinotropic actions preserved in type 2 diabetes | Defective insulinotropic action in type 2 diabetes |
GLP-1
The GLP-1 protein is part of the GCG gene product, which produces five biologically important peptide hormones - glucagon, glucagon-like peptide 1, glucagon-like peptide 2, oxyntomodulin, and glicentin.
Biosynthesis
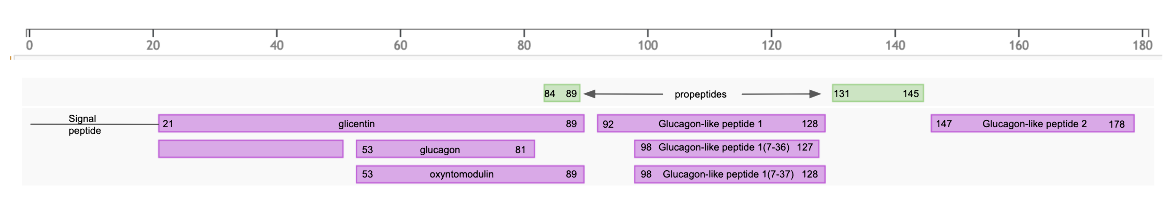
The first 20 amino acids in the translated product is a signal peptide that targets the secretory protein to the rough endoplasmic reticulum (rER) and is removed by signal peptidases. The remaining protein (called proglucagon) is post-translationally processed by various proteases to form the various peptide hormones in a tissue specific manner (see Figure 3).
|
| Figure 3: Post-translational processing of GCG gene product, based on information from UniProt |
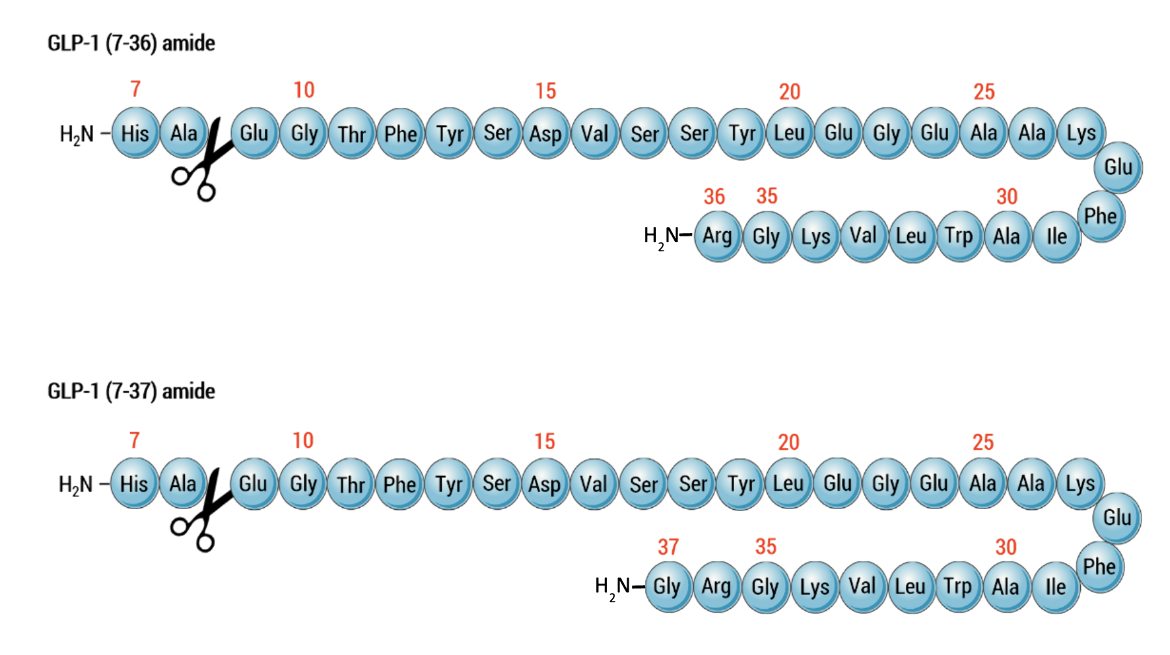
In L-cells of the lower intestine and colon the proprotein convertase subtilisin/kexin type 1 (or PCSK1/PC1) cleaves after single or double basic amino acids to liberate glicentin (amino acids 21-89 in proglucagon), oxyntomodulin (amino acids 53-89 in proglucagon), GLP-1 (amino acids 92-128 in proglucagon), and GLP-2 (amino acids 146-178 in proglucagon) (see UniProt P01275). The first six amino acids at the N-terminus of GLP-1, are further truncated by post-translational processing in the intestinal cells resulting in forming ~31 amino acid peptides - GLP-1(7-37) or GLP-1-(7-36) as shown in Figure 4 below.
The majority of circulating biologically active GLP-1 is found in the GLP-1(7-36) amide form), but a lower concentration of the GLP-1 (7-37) form is detectable. The C-terminal amidation is neither important for the metabolism of GLP-1 nor for its effects on the endocrine pancreas. By multiple mechanisms GLP-1 regulates blood glucose levels, most notably enhancing glucose-dependent synthesis and secretion of insulin in the pancreas. When the protease DPP-4 acts on these peptides to cleave the two N-terminal amino acids, these peptides are rendered inactive.
In pancreatic ɑ-cells, the major bioactive hormone is glucagon that is formed by cleaved by Proprotein Convertase Subtilisin/Kexin Type 2 (PCSK2/PC2).
Structure and Conformation
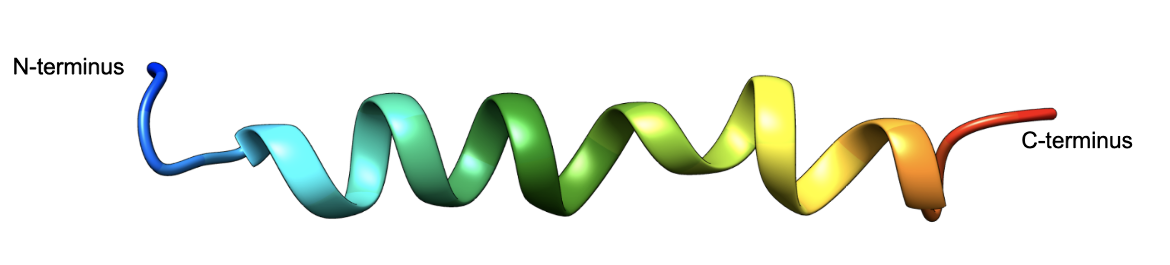
The 31 amino acid peptide forms a helical structure as shown in Figure 5. A majority of the peptide adopts a helical structure in the solvent 2,2,2,-trifluoroethanol (TFE). Experiments show that the C-terminal residues form a helix that extends towards the N-terminus (Chang et al., 2001, see Figure 5). Even when bound to its respective receptor protein GLP-1 adopts a helical structure. Learn more about GLP-1 bound to its receptor.
GIP, the gastric inhibitory polypeptide or the glucose-dependent insulinotropic peptide is released following ingestion of carbohydrates and fats. This hormone binds to specific G-protein-coupled receptors on pancreatic β-cells called the GIP receptor. The incretin effect is exclusively glucose-dependent and critical for the maintenance of glucose homeostasis. Although GIP does not interact with pancreatic α-cells, it has myriad physiological functions, including enhancing glucose-dependent insulin secretion during the postprandial phase, increasing β-cell proliferation, and antiapoptotic and neogenetic effect on the β-cells. In response to hyperglycemia, GIP exhibits no effect on glucagon responses while potentiating insulin secretion. In contrast, during fasting and hypoglycemic conditions, GIP acts to increase glucagon secretion with little effect on insulin release. Therefore, GIP functions as a bifunctional regulator that stabilizes blood glucose and has diverging, glucose-dependent effects on the two main pancreatic glucoregulatory hormones (Christensen et al., 2011). Since GIP’s may also enhance glucagon secretion, it has little pharmacological significance and thus considered not useful as a pharmacological agent for Diabetes treatment.
|
| Figure 5. The 3D structure of Structure of GLP-1 (7-36)-amide in Trifluoroethanol (PDB ID 1d0r, Chang et al., 2001), shown in ribbon representation, colored using the rainbow color scheme with the N-terminus shown in blue and the C-terminus in red. |
GIP
Biosynthesis
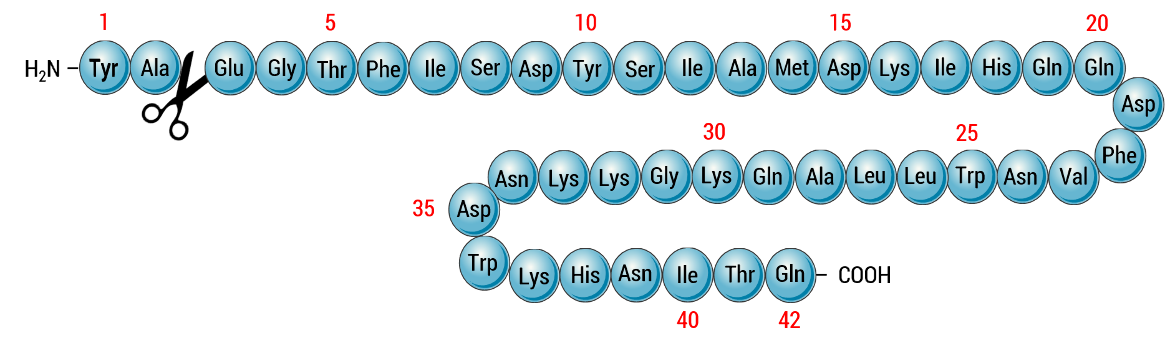
GIP is synthesized and secreted from K cells, which are found primarily in the epithelium of the duodenum and the proximal jejunum (Silberstein, 2013). The GIP gene product has a 21 amino acid signal peptide (UniProt P09681) that targets the protein to rough endoplasmic reticulum (rER) and is cleaved by signal peptidases. The prohormone is further post-translationally processed by prohormone convertases that cleave the gene product after basic amino acids to produce the 42 amino acid GIP hormone (amino acids 52-93) (see Figure 6).

|
| Figure 6: Post-translational processing of GCG gene product, based on information from UniProt |
The study on GIP’s physiological function revealed this hormone modulates glucagon and insulin secretion in a manner that is highly glucose-dependent. When the protease DPP-4 acts on these peptides to cleave the two N-terminal amino acids, these peptides are rendered inactive (Figure 7).
Structure and Conformation
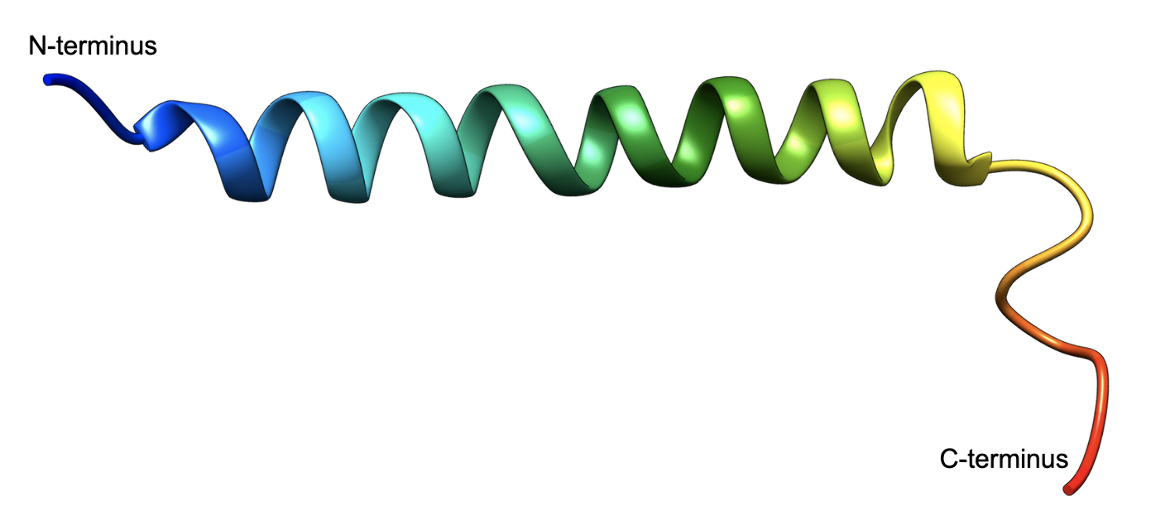
The 42 amino acid peptide forms a helical structure as shown in Figure 8. A majority of the peptide adopts a helical structure in water and the solvent 2,2,2,-trifluoroethanol (TFE) (PDB ID 2obu, Alana et al., 2007). The hormone adopts an overall helical structure in micelles (Venneti et al., 2011) as well as when bound to its respective receptor protein (Sun et al., 2022). Learn more about GIP bound to its receptor.
|
| Figure 8. The 3D structure of GIP in Trifluoroethanol/water (PDB ID 2obu, Alana et al., 2007), shown in ribbon representation, colored using the rainbow color scheme with the N-terminus shown in blue and the C-terminus in red. |
Incretin Function
A summary of physiological functions of GLP-1 and GIP is shown in Figure 9.
Incretin Analogs
Incretins synthesized in our body are rapidly inactivated within a few minutes by the action of the endogenous protease DPP-4. To use incretins therapeutically, they would have to be injected continuously (Nauck et al., 1996). Studies have shown that N-terminal modifications of Incretins - specifically changing the second amino acid in the GLP-1 and GIP molecules from Ala to Gly or other amino acid can reduce the rate of DPP-4 inactivation of these peptides without impacting their ability to bind to their cognate receptors (Green and Flatt, 2007). A peptide isolated from the saliva of a poisonous lizard, Gila monster (Heloderma suspectum), native to the US and Mexico shares 50% amino acid sequence identity with human GLP-1. This protein, Exendin-4, naturally has Glycine as the second amino acid, making it resistant to DPP-4 inactivation. A synthetic form of this peptide, called Exenatide, was the first incretin analog approved by the US FDA to treat diabetes. Since then several other synthetic incretin analogs (e.g., Liraglutide, Semaglutide, and Trizepatide) have been developed with half-lives between 12 hours and 3-4 days. These are now available as once-daily or weekly injectable incretin analogs for therapeutic use. In addition to blood glucose level management, many of these have been shown to be effective in lowering weight.
References
Alaña, I., Malthouse, J.P., O'Harte, F.P., Hewage, C.M. (2007) The bioactive conformation of glucose-dependent insulinotropic polypeptide by NMR and CD spectroscopy. Proteins. 68, 92-9. https://doi.org/10.1002/prot.21372
Christensen, M., Vedtofte, L., Holst, J.J., Vilsbøll, T., Knop, F. K. (2011) Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 60, 3103-9. https://doi.org/10.2337/db11-0979
Green, B.D., Flatt, P.R. (2007) Incretin hormone mimetics and analogues in diabetes therapeutics. Best Pract Res Clin Endocrinol Metab. 21, 497-516. https://doi.org/10.1016/j.beem.2007.09.003
Joy SV, Rodgers PT, Scates AC. (2005) Incretin mimetics as emerging treatments for type 2 diabetes. Ann Pharmacother. 39, 110-118, https://doi.org/10.1345/aph.1e245
Nauck, M.A., Wollschläger, D., Werner, J., Holst, J.J., Orskov, C., Creutzfeldt, W., Willms, B. (1996) Effects of subcutaneous glucagon-like peptide 1 (GLP-1 [7-36 amide]) in patients with NIDDM. Diabetologia. 39, 1546-53. https://doi.org/10.1007/s001250050613
Norman, A.W. and Henry, H.L. (2015). "Gastrointestinal Hormones". Academic Press: 141-169. https://doi.org/10.1073/pnas.0706404104
Rutter, G.A. and Zac-Varghese, S. (2016) Incretin Biology, 1st ed. Singapore: Imperial College Press, Print.
Seino, Y., Fukushima, M., and Yabe, D. (2010). "GIP and GLP-1, the Two Incretin Hormones: Similarities and Differences". Journal of Diabetes Investigation, 1(1.5): 8-23. https://doi.org/10.1111/j.2040-1124.2010.00022.x
Silberstein, I. (2013). What Is The Role of Incretin Mimetics In The Treatment of Type 2 Diabetes?. The Science Journal of the Lander College of Arts and Sciences, 7(1). Retrieved from https://touroscholar.touro.edu/sjlcas/vol7/iss1/2
Sun, B., Willard, F.S., Feng, D., Alsina-Fernandez, J., Chen, Q., Vieth, M., Ho, J.D., Showalter, A.D., Stutsman, C., Ding, L., Suter, T.M., Dunbar, J.D., Carpenter, J.W., Mohammed, F.A., Aihara, E., Brown, R.A., Bueno, A.B., Emmerson, P.J., Moyers, J.S., Kobilka, T.S., Coghlan, M.P., Kobilka, B.K., Sloop, K.W. (2022) Structural determinants of dual incretin receptor agonism by tirzepatide. Proc Natl Acad Sci U S A. 119, e2116506119. https://doi.org/10.1073/pnas.2116506119
Venneti, K.C., Malthouse, J.P., O'Harte, F.P., Hewage, C.M. (2011) Conformational, receptor interaction and alanine scan studies of glucose-dependent insulinotropic polypeptide, Biochim Biophys Acta. 1814, 882-8. https://doi.org/10.1016/j.bbapap.2011.04.002
Yamada, Y., Miyawaki, K., Tsukiyama, K., Harada, N., Yamada, C., Seino, Y. (2006) Pancreatic and Extrapancreatic Effects of Gastric Inhibitory Polypeptide. Diabetes 55 (Supplement_2): S86–S91. https://doi.org/10.2337/db06-S011
August 2023 Jennifer Jiang and Dr. Shuchismita Dutta; Reviewed by Dr. Joseph D. Ho
http://dx.doi.org/10.2210/rcsb_pdb/GH/DM/drugs/in/Incretins